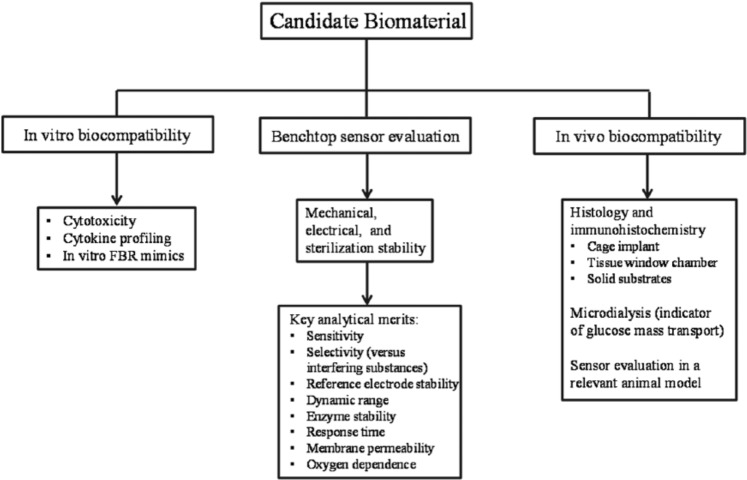
Figure 1.
Noninclusive flow diagram for initial testing of biocompatible glucose sensor coatings. In vitro biocompatibility assays may be carried out to test for material toxicity, surface biofouling, and cell phenotype.7 Results from such assays generally help identify promising candidate materials for further study. Benchtop sensor evaluation should assess the stability of the final device and analytical performance in both physiological buffer and complex media (eg, serum, whole blood). In vivo analysis should only be pursued after in vitro studies and should examine several tissue characteristics (histology, tissue glucose transport, etc). Sensor evaluation in a relevant animal model represents the ultimate step in this process and may be used to examine any potential benefits to CGM performance identified in prior studies.