Abstract
Skeletal muscle is mainly composed of myofibers and intramuscular connective tissue. Bundles composed of many myofibers, with each myofiber sheathed in connective tissue called the endomysium, are packed in the perimysium, which occupies the vast bulk of the intramuscular connective tissue. The perimysium is a major determination factor for muscle texture. Some studies have reported that collagen peptide (Col-Pep) ingestion improves the connective tissue architecture, such as the tendon and dermis. The present study evaluated the effects of Col-Pep ingestion on the chicken iliotibialis lateralis (ITL) muscle. Chicks were allocated to three groups: the 0.15% or 0.3% Col-Pep groups and a control group. Col-Pep was administered by mixing in with commercial food. On day 49, the ITL muscles were analyzed by morphological observation and the textural property test. The width of the perimysium in the 0.3% Col-Pep group was significantly larger than other two groups. Although scanning electron microscopic observations did not reveal any differences in the architecture of the endomysium, elastic improvement of the ITL muscle was observed as suggested by an increase of the width of perimysium and improved rheological properties. Our results indicate that ingestion of Col-Pep improves the textural property of ITL muscle of chickens by changing structure of the perimysium.
Keywords: chicken, collagen peptide, perimysium, skeletal muscle, textural property
Skeletal muscle is mainly composed of myofibers and intramuscular connective tissue. Each myofiber is sheathed in a connective tissue called the endomysium, and the individual myofibers are gathered together to make a muscle bundle. The bundle is packed in the perimysium, which occupies the vast bulk of the intramuscular connective tissue [19]. It is suggested that collagens, the major protein constituent of the perimysial and endomysial connective tissues [4, 20], are the major determinant of muscle texture, primarily influencing the muscle quality, rather than the muscle quantity. Skeletal muscle contains several types of collagens, such as types I, III, IV, V and VI collagens [18], but the collagen constituting the perimysium and endomysium of skeletal muscle is mainly type I collagen [1, 3, 20].
The iliotibialis lateralis (ITL) muscle, which is located in the femoral superficial muscles and has another name of biceps femoris muscle, has been used to evaluate the quality of meat produced from the thigh muscle of chickens [2]. The ITL muscle of chicken is a large unipennate muscle that plays an important role in locomotion, and the collagen fiber structure in the ITL muscle is well developed under severe environmental conditions [2, 15]. It has also been reported that differences in nutritional levels in the diet lead to morphological differences in the perimysium and endomysium of the broiler ITL muscle [2].
Heat-denatured collagen extracted from animal hide, bone or fish scale is referred to as gelatin. Collagen peptide (Col-Pep) is prepared by limited digestion of gelatin and is widely used as an ingredient of food or supplement. Minaguchi et al. reported that ingestion of Col-Pep led to significant increases in the diameter and density of collagen fibrils in the rabbit Achilles tendon in a dose-dependent manner [14]. Similarly, Matsuda et al. reported that ingestion of Col-Pep induced significant increases in the diameter and density of collagen fibrils in the porcine dermis [13]. These studies suggested that Col-Pep ingestion modulates the structure and function of the connective tissue, such as the Achilles tendon and the dermis that is mainly composed of type I collagen as is the case for the fascia. In the present study, effects of Col-Pep ingestion on the morphological characteristics of the perimysium and endomysium in the broiler ITL muscle were investigated, and the change in the textural property of the ITL muscle after Col-Pep ingestion was investigated by a textural property test.
MATERIALS AND METHODS
Animals and feed: Chickens were treated in accordance with the “Guideline for Regulation of Animal Experimentation (1997)” of the Faculty of Agriculture, Shinshu University. A total of 45 male broiler chicks (Gallus gallus domestics; Ross 308, Avaigen) at 1-day of age were commercially obtained from Komatsu Shukeijo (Matsumoto, Japan) and used for the 49-day study. The chicks were maintained in a room with 24-hr lighting at a temperature of 32°C for 7 days and provided with a commercial starter food (Nippai Co., Ltd., Yokohama, Japan) and water ad libitum. The chicks were weighed on day 7 and allocated to three floor pens (1.8 × 0.9 m; 15 birds/pen), such that the average body weight did not differ among the groups. The body weights on day 7 in the control, 0.15% Col-Pep and 0.3% Col-Pep groups were 160.45 ± 11.6, 159.5 ± 11.5 and 159.6 ± 10.5 g (mean ± standard deviation), respectively. The pens contained recycled litter of corrugated cardboard and wood shavings on a concrete floor. After this allocation, the chicks remained with ad libitum access to water and feed. The three groups were fed the starter food for the first 21 days after allocation and thereafter fed a commercial finisher food (Nippai Co., Ltd.). The control group was fed the diet of starter and finisher foods only. The Col-Pep groups were administered food containing 0.15% or 0.3% Col-Pep (MW: 4,000–6,000; nitrogen content: 16.9%; fish-derived; Nippi Inc., Tokyo, Japan) such that a daily Col-Pep intake of 0.1 or 0.2 g/kg body weight was attained in the finisher period, respectively. The dose of 0.2 g/kg body weight was selected based on the effect reported in the porcine skin (62-day intake), the mouse skin (56-day intake), human skin (42-day intake), the rabbit Achilles tendon (56-day intake) and the rat blood lipids (29-day intake) [9, 10, 13, 14, 21]. To examine whether the effect of Col-Pep ingestion was collagen-specific or caused by ingestion of protein in general, the crude protein levels were adjusted to approximately equal amounts (days 0–21: 23.5%; days 22–49: 18.0%) using maize (Marui-Sangyo Co., Ltd., Nagano, Japan). The body weight of all birds was measured every week. On day 49, ten birds close to the average body weight in each group were selected for sample collection and euthanized by bleeding. The carcasses were divided into six and four samples so that body weight did not differ significantly among all groups. The ITL muscles from six carcasses were prepared for morphological observation, and the other four samples were stored at 4°C for 24 hr until use in the textural property test. The remaining samples after property test were taken off the epimysium and stored at −20°C until used for the quantification of total collagen content.
Light microscopy: Samples (10 × 10 × 10 mm) with parallel myofiber striation were obtained from the central zone of each ITL muscle, fixed in Zamboni’s fixative, containing 4% paraformaldehyde in 15% saturated picric acid and 0.1M phosphate buffer (pH 7.4), for 24 hr at 4°C, washed several times with 0.1M phosphate buffer and embedded in paraffin wax according to standard procedures. Transverse paraffin sections were cut at a thickness of 5 µm and mounted on silane-coated glass slides. Deparaffinized sections were stained by the Masson trichrome method. Sixteen tissue sections from one ITL muscle (96 tissue sections/6 birds/group) were randomly selected. The perimysium was classified into a thin perimysium composing the primary myofiber fascicle and a thick perimysium composing the secondary myofiber fascicle. A total of 384 thick and thin perimysium widths in each group were measured as follows. Four thick and thin perimysium widths were randomly selected on one tissue section, and the width of the thick perimysium was measured at a position that did not include blood vessels (Fig. 1).
Fig. 1.
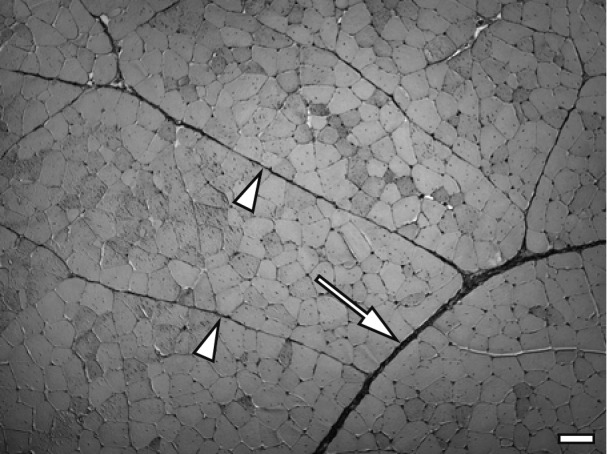
Masson trichrome staining for the thick perimysium (arrow) and thin perimysium (arrowheads) in the ITL muscle of control chicken on day 49. Bar=100 µm.
Scanning electron microscopy (SEM): Samples (5 × 5 × 10 mm) from the same region used for the light microscopic observation were fixed in Karnovsky’s fixative, containing 2% paraformaldehyde and 2.5% glutaraldehyde in 0.1M phosphate buffer (pH 7.4), for 24 hr at 4°C. After rinsing with 0.1M phosphate buffer for 24 hr, the samples were macerated in 2M NaOH for 4 days, rinsed with distilled water, post-fixed in 1% osmium tetroxide in 0.1M phosphate buffer (pH 7.4) for 1 hr at room temperature, rinsed again with distilled water and dehydrated through graded concentrations of ethanol. For SEM observation, samples were dried by the t-butyl alcohol freeze-drying method [8], mounted on metal stubs and coated with osmium using an osmium coater (IB; Eiko, Hitachinaka, Japan). Each endomysium was observed under a SEM (JSM-5200; JEOL, Tokyo, Japan) with an accelerating voltage of 5 kV.
Measurement of textural properties: A creep meter (RE2-3305S; Yamaden, Tokyo, Japan) was used to measure the failure stress (the limit at which the sample broke under outside pressure) and the strain at failure of the ITL muscle. The testing conditions were as follows: room temperature: 25 ± 1°C; sample size: 10 × 10 × original height in centimeters; plunger: cylindrical form (50-mm diameter); measurement strain rate: 100%; compression speed: 1.0 mm/sec. The test conducted the compression stress for 5 points per one sample to improve reproducibility. The peak top point of the stress–strain curve was defined as the failure point of the sample. A photograph of the structural property test and a typical stress–strain curve measured by the creep meter are shown in Fig. 2a and 2b, respectively.
Fig. 2.
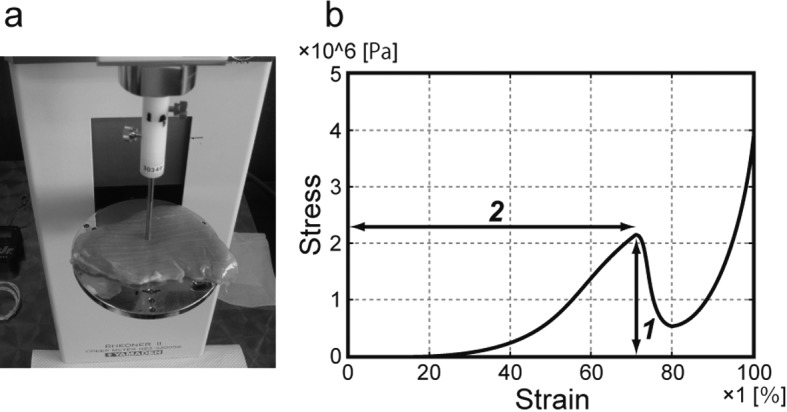
Photograph of the creep meter (a) and a typical stress–strain curve obtained by the creep meter (b). In (a), the ITL muscle is mounted on a plate and loaded compression stress using a plunger. In (b), ‘1’ indicates the failure stress, and ‘2’ indicates the strain at failure.
Quantification of total collagen content: The quantification of total collagen content included in ITL muscle was carried out at Japan Institute of Leather Research (Toride, Japan). The 0.2 g of sample was added in 10 ml of 6N HCl and hydrolyzed for 24 hr at 110°C. After having removed hydrochloric acid by vacuum concentration, the sample was dissolved in 10 ml of distilled water, and 10 µl was subjected to analysis by an amino acid analyzer (Hitachi L-800; Hitachi, Japan). The total collagen content was calculated from the quantity of hydroxyproline, a marker amino acid of collagen.
Statistical analyses: Differences among live weights, perimysium widths, failure stress, strain at failure and total collagen content were compared between the control, 0.15% Col-Pep and 0.3% Col-Pep groups using ANOVA and Tukey–Kramer test as multiple comparisons. The significance level (P) was set at 0.05.
RESULTS
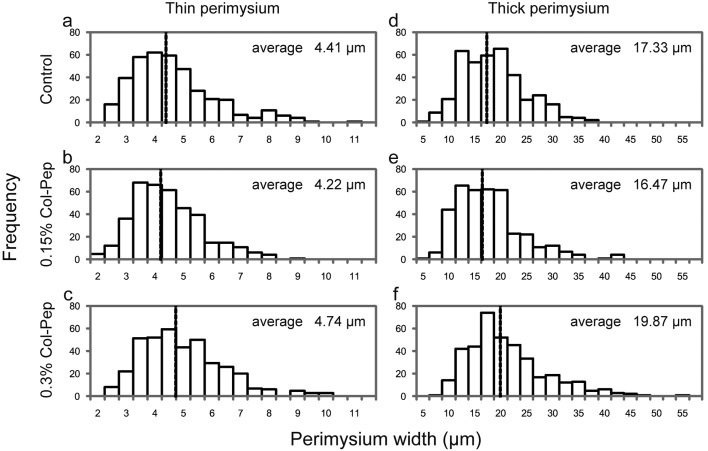
Throughout the feeding period, no significant differences were observed in the body weights among the experimental groups. The body weights at the end of the feeding period in the control, 0.15% Col-Pep and 0.3% Col-Pep groups were 3,275.0 ± 427.3, 3,426.7 ± 338.9 and 3,593.3 ± 174.0 g (mean ± standard deviation), respectively, and increased in a dose-dependent manner of Col-Pep. The effects of Col-Pep on the thick and thin perimysium widths are summarized in Table 1. The widths of the perimysium, both thick and thin, were significantly larger in the 0.3% Col-Pep group than in the other two groups. Figure 3 shows histograms of the thin and thick perimysia in the three groups.
Table 1. Perimysium widths and collagen content in the ITL muscle.
| Chicken groups | ||||
|---|---|---|---|---|
| Control | 0.15% Col-Pep | 0.3% Col-Pep | ||
| Perimysium width (µm) | ||||
| Thick perimysium | 4.41 ± 1.5a | 4.22 ± 1.2a | 4.74 ± 1.4b | |
| style="pading-left:10pt"Thin perimysium | 17.33 ± 6.1a | 16.47 ± 6.5a | 19.87 ± 7.6b | |
| Collagen content (mg/g) | 1.51 ± 0.18 | 1.67 ± 0.20 | 1.64 ± 0.16 | |
Data are shown as the mean ± standard deviation. a,b: Significant differences among the three groups (P<0.01).
Fig. 3.
Histograms of thin perimysium width (a–c) and thick perimysium width (d–f) in the ITL muscle of the control (a, d), 0.15% Col-Pep (b, e) and 0.3% Col-Pep (c, f) groups. Dotted line in the histogram represents average width.
SEM observation under a low magnification revealed the honeycomb structure of the endomysium and the perimysium band composed of collagen fibers (Fig. 4a–4c). Observation under a high magnification did not reveal any differences in the fundamental architectures of the endomysium wall, such as the collagen fibril orientation or density, among the groups (Fig. 4d–4f).
Fig. 4.
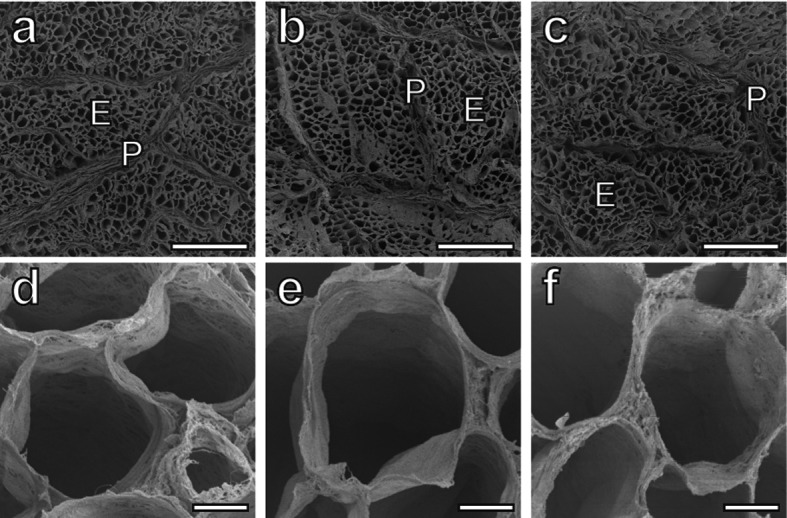
(a–c) Low-magnification SEM photographs of the perimysium (P) around the primary myofiber fascicle and the endomysium (E) of ITL muscles from the control (a), 0.15% Col-Pep (b) and 0.3% Col-Pep (c) groups. Bars=500 µm. (d–f) High-magnification SEM photographs of the endomysium wall of ITL muscles from the control (d), 0.15% Col-Pep (e) and 0.3% Col-Pep (f) groups. Bars=20 µm.
The measurement results for the textural properties are summarized in Table 2. The failure stress increased in the order of the control group, 0.15% Col-Pep group and 0.3% Col-Pep group, but there were no significant differences. The strain at failure was significantly increased in the 0.3% Col-Pep group compared with the control group.
Table 2. Rheological properties of the ITL muscle.
| Chicken groups | |||
|---|---|---|---|
| Control | 0.15% Col-Pep | 0.3% Col-Pep | |
| Failure stress (103 Pa) | 1546 ± 425 | 1658 ± 255 | 1712 ± 360 |
| Strain at failure (%) | 60.17 ± 7.23a | 63.70 ± 9.39a,b | 68.61 ± 5.83b |
Data are shown as the mean ± standard deviation. a,b: Significant differences among the three groups (P<0.01).
No significant differences were observed in the total collagen content among all groups regardless of the perimysium width and textural property (Table 1).
DISCUSSION
This is the first study to analyze effects of Col-Pep ingestion on perimysium and endomysium and the change in the textural property of the skeletal muscle. The ITL muscle is located within a wide range of the thigh muscles and is composed of red fast-twitch myofibers with high oxidative activity [2, 7]. ITL muscle was used in this study to evaluate the quality of meat obtained from the thigh muscle of chickens, because ITL meat is preferred in Japan over breast meat that is composed of white fast-twitch myofibers with low oxidative activity. Iwamoto et al. reported that the ITL muscle shows a positive allometric growth relationship with body weight [6]. Roy et al. revealed that the body weight and ITL muscle weight of broiler chickens reared under a high nutrient condition were significantly higher than those in birds reared under a low nutrient condition and that both the thick and thin perimysium widths were also significantly increased under the high nutrient condition [19]. However, they also reported that the total collagen content of the ITL muscle did not differ between high and low nutrient conditions regardless of perimysium widths. Thus, it was suggested that perimysium widths do not correlate with the total collagen content of the ITL muscle.
In the present study, the crude protein content of the diet in all three groups was adjusted to be approximately equal, and no significant differences were observed in the body weights among the experimental groups. Under this experimental condition, the widths of the perimysium, both thick and thin, were significantly higher in the 0.3% Col-Pep group than in the other two groups. These results suggest that prolonged ingestion of 0.3% Col-Pep increased the width of the perimysium in broiler ITL muscle irrespective of body weight gain. Because the total collagen content did not differ significantly among the three groups, it is also suggested that perimysium widths do not correlate with the total collagen content of the ITL muscle as was reported by Roy et al. [19].
In contrast to the perimysium, the morphological structures of the endomysium did not differ among the three groups. Nakamura et al. revealed that the accumulation of collagen fibrils in the endomysium of the ITL muscle increased gradually according to age [16], and Das et al. suggested that the cell size of the endomysial honeycomb increased according to the developmental stage [2]. However, Roy et al. reported that differences in nutrient conditions did not cause any differences in the endomysium wall architecture composed of fine collagen bundles or fibrils in broiler ITL muscle [20]. Although the endomysium contains type I collagen [11, 18] as the Achilles tendon which exhibits significant changes upon Col-Pep ingestion in rabbits [14], the present study suggests that the endomysium of the ITL muscle is not the connective tissue affected by Col-Pep ingestion.
It is well known that collagen is an important factor related to the development of meat toughness. Liu et al. reported a significant correlation between meat toughness of raw chicken and the total collagen content [12]. Nishimura et al. described that the width of the thick perimysium determines the mechanical strength and contributes to the toughness in raw pork [17]. However, the above two studies were carried out by comparisons with different muscles. It was also revealed that meat toughness increased with age, owing to changes in the chemical and architectural properties of collagen content regardless of decreases in the quantity of collagen [2, 5, 22]. In the present study, the index of ITL muscle toughness was divided into two textural properties, failure stress and strain at failure, for analysis. The results clarified that the thickened perimysium induced by 0.3% Col-Pep ingestion resulted in a textural property change of the ITL muscle in chickens with the same age. The change was not expressed as an increase in hardness (failure stress), but as an increase in elasticity (strain at failure). The perimysium develops in the muscle of a body part with intense exercise and is thought to contribute to both transmission of the muscular contraction power and maintenance of the muscle structure [17]. In chickens, the ITL muscle plays an important role in the production of propelling power. Therefore, the present study suggests that the thickening of the perimysium observed with 0.3% Col-Pep ingestion and the increase in its elasticity would improve both muscle contraction power and muscle structure maintenance. However, this suggestion is not without limitation. Though no significant differences were observed in the diameters of the myofibers under light microscopic observation among the experimental groups (data is not shown), possibilities of the effect of more sophisticated myofiber morphology, such as change of myofiber types or electron microscopic characteristics, were not considered about elastic improvement in the present study. Yet, this is the first study to elucidate effect of Col-Pep ingestion on perimysium. Although Col-Pep used in the study is solubilized and partially digested with enzymes, collagen contained in commercial chicken food is not subjected to such processes. The fact that effect of Col-Pep ingestion was observed in the present study provided possibility that digestibility and absorptivity in gastrointestinal tract of collagen included in the commercial chicken food may be different from those of Col-Pep.
In conclusion, the present study revealed that 0.3% Col-Pep ingestion significantly increased the perimysium width of the ITL muscle in chickens, but did not affect the endomysium architecture. This morphological change in the muscle was confirmed as elastic improvement by the textural property examination. Because significant effects of Col-Pep ingestion were observed on the skin and the Achilles tendon in mammals [10, 13, 14, 21], it seems that Col-Pep ingestion modulates structure and function of collagen-rich tissues in wide range of species including mammals and bird.
Acknowledgments
We wish to thank Prof. K. Koh (Shinshu University) and Prof. D. Suzuki (Sapporo Medical University) for their technical supports in this study.
REFERENCES
- 1.Bailey A. J., Light N. D.1989. Connective Tissue in Meat and Meat Products. Elsevier Applied Science, London. [Google Scholar]
- 2.Das C., Roy B. C., Oshima I., Miyachi H., Nishimura S., Iwamoto H., Tabata S.2009. Collagen content and architecture of the Iliotibialis lateralis muscle in male chicks and broilers with different growth rates fed on different nutritional planes. Br. Poult. Sci. 50: 47–56. doi: 10.1080/00071660802613294 [DOI] [PubMed] [Google Scholar]
- 3.Das C., Roy B. C., Oshima I., Miyachi H., Nishimura S., Iwamoto H., Tabata S.2010. Collagen content and architecture of the pectoralis muscle in male chicks and broilers reared under various nutritional conditions. Anim. Sci. J. 81: 252–263. doi: 10.1111/j.1740-0929.2009.00730.x [DOI] [PubMed] [Google Scholar]
- 4.Dransfield E., Sosnicki A. A.1999. Relationship between muscle growth and poultry meat quality. Poult. Sci. 78: 743–746. doi: 10.1093/ps/78.5.743 [DOI] [PubMed] [Google Scholar]
- 5.Fang S. H., Nishimura T., Takahashi K.1999. Relationship between development of intramuscular connective tissue and toughness of pork during growth of pigs. J. Anim. Sci. 77: 120–130. [DOI] [PubMed] [Google Scholar]
- 6.Iwamoto H., Hara Y., Gotoh T., Ono Y., Takahara H.1993. Different growth rates of male chicken skeletal muscles related to their histochemical properties. Br. Poult. Sci. 34: 925–938. doi: 10.1080/00071669308417653 [DOI] [PubMed] [Google Scholar]
- 7.Iwamoto H., Katoh F., Gotoh T., Nishimura S., Ono Y., Nishio Y., Fukuhara E., Murakami T.1998. Effects of parent Shamo cocks on the histochemical properties of M-iliotibialis lateralis and M-supracoracoideus on their crossbred broilers. Br. Poult. Sci. 39: 589–595. doi: 10.1080/00071669888449 [DOI] [PubMed] [Google Scholar]
- 8.Iwasaki S., Hosaka Y., Iwasaki T., Yamamoto K., Nagayasu A., Ueda H., Kokai Y., Takehana K.2008. The mudulation of collagen fibril assembly and its structure by decorin: an electron microscopic study. Arch. Histol. Cytol. 71: 37–44. doi: 10.1679/aohc.71.37 [DOI] [PubMed] [Google Scholar]
- 9.Koyama Y., Kusubata M.2013. Effects of collagen peptide ingestion on blood lipids in rats fed a high-lipid and high-sucrose diet. Food Sci. Technol. Res. 19: 1149–1153. doi: 10.3136/fstr.19.1149 [DOI] [Google Scholar]
- 10.Koyama Y., Kuwaba K., Kondo S., Tsukada Y.2014. Supplemental ingestion of fish-derived collagen peptide suppresses ultraviolet-induced erythema: A randomized double-blind placebo-controlled study. Jpn. Pharmacol. Ther. 42: 781–790. [Google Scholar]
- 11.Listrat A., Picard B., Geay Y.1999. Age-related changes and location of type I, III, IV, V and VI collagens during development of four foetal skeletal muscles of double-muscled and normal bovine animals. Tissue Cell 31: 17–27. doi: 10.1054/tice.1998.0015 [DOI] [PubMed] [Google Scholar]
- 12.Liu A., Nishimura T., Takahashi K.1996. Relationship between structural properties of intramuscular connective tissue and toughness of various chicken skeletal muscles. Meat Sci. 43: 43–49. doi: 10.1016/0309-1740(95)00065-8 [DOI] [PubMed] [Google Scholar]
- 13.Matsuda N., Koyama Y., Hosaka Y., Ueda H., Watanabe T., Araya T., Irie S., Takehana K.2006. Effects of ingestion of collagen peptide on collagen fibrils and Glycosaminoglycans in the dermis. J. Nutr. Sci. Vitaminol. (Tokyo) 52: 211–215. doi: 10.3177/jnsv.52.211 [DOI] [PubMed] [Google Scholar]
- 14.Minaguchi J., Koyama Y., Meguri N., Hosaka Y., Ueda H., Kusubata M., Hirota A., Irie S., Mafune N., Takehana K.2005. Effects of ingestion of collagen peptide on collagen fibrils and glycosaminoglycans in Achilles tendon. J. Nutr. Sci. Vitaminol. (Tokyo) 51: 169–174. doi: 10.3177/jnsv.51.169 [DOI] [PubMed] [Google Scholar]
- 15.Nakamura Y. N., Iwamoto H., Tabata S., Ono Y., Shiba N., Nishimura S.2003. Comparison of collagen content, distribution and architecture among the pectoralis, iliotibialis lateralis and puboischiofemoralis muscles with different myofiber composition in Silky cocks. Anim. Sci. J. 74: 119–128. doi: 10.1046/j.1344-3941.2003.00096.x [DOI] [Google Scholar]
- 16.Nakamura Y. N., Iwamoto H., Shiba N., Miyachi H., Tabata S., Nishimura S.2004. Growth changes of the collagen content and architecture in the pectoralis and iliotibialis lateralis muscles of cockerels. Br. Poult. Sci. 45: 753–761. doi: 10.1080/00071660400014309 [DOI] [PubMed] [Google Scholar]
- 17.Nishimura T., Fang S., Wakamatsu J., Takahashi K.2009. Relationships between physical and structural properties of intramuscular connective tissue and toughness of raw pork. Anim. Sci. J. 80: 85–90. doi: 10.1111/j.1740-0929.2008.00600.x [DOI] [PubMed] [Google Scholar]
- 18.Nishimura T., Ojima K., Hattori A., Takahashi K.1997. Developmental expression of extracellular matrix components in intramuscular connective tissue of bovine semitendinosus muscle. Histochem. Cell Biol. 107: 215–221. doi: 10.1007/s004180050106 [DOI] [PubMed] [Google Scholar]
- 19.Roy B. C., Oshima I., Miyachi H., Shiba N., Nishimura S., Tabata S., Iwamoto H.2006. Effects of nutritional level on muscle development, histochemical properties of myofibre and collagen architecture in the pectoralis muscle of male broilers. Br. Poult. Sci. 47: 433–442. doi: 10.1080/00071660600828334 [DOI] [PubMed] [Google Scholar]
- 20.Roy B. C., Oshima I., Miyachi H., Shiba N., Nishimura S., Tabata S., Iwamoto H.2007. Histochemical properties and collagen architecture of M-iliotibialis lateralis and M-puboischiofemoralis in male broilers with different growth rates induced by feeding at different planes of nutrition. Br. Poult. Sci. 48: 312–322. doi: 10.1080/00071660701370491 [DOI] [PubMed] [Google Scholar]
- 21.Shibuya S., Ozawa Y., Toda T., Watanabe K., Tometsuka C., Ogura T., Koyama Y., Shimizu T.2014. Collagen peptide and vitamin C additively attenuate age-related skin atrophy in Sod1-deficient mice. Biosci. Biotechnol. Biochem. 78: 1212–1220. doi: 10.1080/09168451.2014.915728 [DOI] [PubMed] [Google Scholar]
- 22.Wojtysiak D.2013. Effect of age on structural properties of intramuscular connective tissue, muscle fibre, collagen content and meat tenderness in pig longissimus lumborum muscle. Folia Biol. (Krakow) 61: 221–226. doi: 10.3409/fb61_3-4.221 [DOI] [PubMed] [Google Scholar]