SUMMARY
We compared phenotypes of five strains of Mycobacterium tuberculosis (Mtb) differing in their expression of rv1248c and its product, 2-hydroxy-3-oxoadipate synthase (HOAS), with a focus on carbon source-dependent growth rates and attenuation in mice. Surprisingly, an rv1248c transposon mutant on a CDC1551 background grew differently than an rv1248c deletion mutant on the same background. Moreover, the same rv1248c deletion in two different yet genetically similar strain backgrounds (CDC1551 and H37Rv) gave different phenotypes, though each could be complemented. Whole genome re-sequencing did not provide an obvious explanation for these discrepancies. These observations offer a cautionary lesson about the strength of inference from complementation and sequence analysis, and commend consideration of more complex phenomena than usually contemplated in Mtb, such as epigenetic control.
Keywords: Genetic complementation, whole genome sequencing, transposon mutagenesis, CDC1551, hyroxyoxoadipate synthase
1. Introduction
As tuberculosis (TB) continues to impose a devastating global burden, drug discovery efforts put a premium on characterization of annotated and orphan genes implicated in pathways vital for survival of Mycobacterium tuberculosis (Mtb). [1]. Transposon insertion mutagenesis is a powerful tool used to identify genetic elements that are essential for bacterial growth and pathogenesis [2]. Himar-1 transposon mutagenesis libraries generated in an Mtb H37Rv background suggested that rv1248c (hoas), the gene that encodes 2-hydroxy-3-oxoadipate synthase (HOAS), may be essential for in vitro growth [3–5]. HOAS, a thiamin diphosphate (ThDP)-dependent enzyme, functions at a node linking the tricarboxylic acid (TCA) cycle and glutamate synthesis through its substrate, α-ketoglutarate (α-KG). HOAS is multifunctional through its ability to convert α-KG to succinyl-CoA, succinyl semialdehyde (SSA), and hydroxy-oxoadipate (HOA); HOA spontaneously gives rise to 5-hydroxylevulinc acid (HLA) [6]. Recombinant HOAS has been extensively studied in vitro, but characterization of the enzyme’s function in the intact cell requires studies of cells bearing mutant alleles of rv1248c.
A transposon mutant, hoas::tnCDC1551, was generated in a wild-type CDC1551 (WTCDC1551) strain background as part of the Tuberculosis Animal Research and Gene Evaluation Taskforce (TARGET) initiative. Its existence suggested that HOAS is dispensable for growth of Mtb in vitro [7]. CDC1551 caused a TB outbreak near the Kentucky-Tennessee border in the mid 1990s and was initially considered hypervirulent in humans [8]. Studies of CDC1551 in mice found it to be less virulent than [9] or comparable in virulence to the laboratory strain H37Rv [10, 11].
We began assessing the role of HOAS in Mtb by comparing the phenotype of WTCDC1551 and hoas::tnCDC1551. While that work was underway, we used the WTCDC1551 parent to generate the deletion mutant described below, termed ΔhoasCDC1551. We characterized the in vitro growth of ΔhoasCDC1551 and hoas::tnCDC1551 in media with defined carbon sources and compared it to the behavior of the hoas deletion mutant generated in an H37Rv WT background, termed ΔhoasH37Rv, as reported elsewhere [12]. Surprisingly, the phenotypes of all three strains varied, even though all phenotypes but one were reverted to wild type by complementation with a full-length, wild type hoas allele. Whole-genome sequencing identified polymorphisms unique to each mutant and its WT parent but did not offer an apparent explanation for the divergent phenotypes.
2. Materials and methods
2.1 Mtb culture and generation of mutants
Standard liquid and agar culture methods, composition of Sauton’s minimal medium (SMM), and growth experiments in carbon-defined Sauton’s minimal medium were as described [12].
Procedures for engineering ΔhoasH37Rv and its complementation with a full length hoas allele (ΔhoasH37Rv::hoas) were as described [12]. For ΔhoasCDC1551, hoas was cloned downstream of an hsp60 promoter into pDE43-MCS via Gateway Cloning Technology (Invitrogen) to yield pDE43hsp60hoas-MCS, which integrates into the attL5 site. This vector was electroporated into competent WTCDC1551 to create merodiploid WTCDC1551::hoas. This merodiploid strain was transformed with the nitrile-inducible recombineering plasmid pNIT(kan)::RecET-sacB, and colonies were selected on 7H10 agar plates with 10 ug/mL streptomycin and 15 ug/mL kanamycin. Replacement of native hoas with a hygromycin cassette was carried out by methods described in [12]. Knockout candidates (ΔhoasCDC1551::pGMCS-hsp60hoas) were screened for kanamycin sensitivity, implicating loss of pNIT(kan)::RecET-sacB, and native hoas deletion was confirmed by Southern blot. To test essentiality of hoas, a plasmid harboring a zeocin resistance gene, pGMZg170X0X, was transformed into competent ΔhoasCDC::pGMCS-hsp60hoas. ΔhoasCDC1551::pGMZg170X0X (here called ΔhoasCDC1551) candidates were isolated on 7H10 agar with 50 ug/mL hygromycin and 25 ug/mL zeocin and further screened by immunoblot. Plasmids pNIT(kan)::RecET-sacB, pDE43-MCS, and pGMZg170X0X were a kind gift from Dirk Schnappinger, Weill Cornell Medical College.
2.2 Other procedures
Protocols for immunoblotting and Mtb aerosol infection of mice were as reported in [12].
2.3 Whole Genome Sequencing
The genomes of the five strains described in this study were sequenced using next-generation sequencing technology. Three of the strains were sequenced using an Illumina HiSeq 2500 with a read length of 54 bp, and the other two were sequenced using an Illumina MiSeq with a read length of 150 bp. The machines were operated in paired end mode. All samples were prepared according to the standard Illumina genomic DNA sample preparation protocol (Illumina, Inc.). The sequencing statistics, including read-length and depth of coverage for each sample are given in Table S1. Genome sequences were assembled by a comparative method as described in [13], using published genomes for H37Rv and CDC1551 as reference sequences.
3. Results
3.1 Generation and complementation of hoas mutant strains on the CDC1551 background
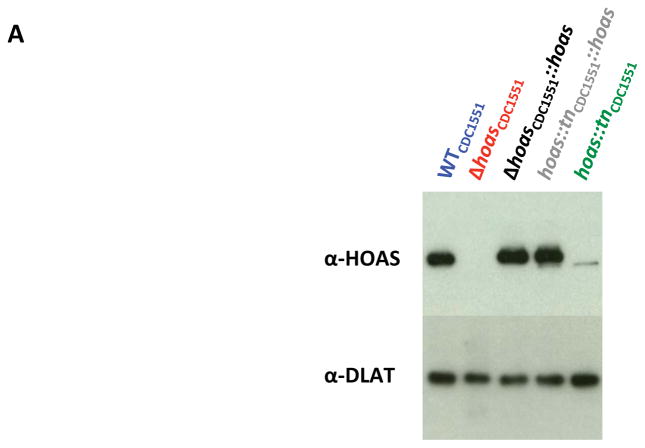
Immunoblot with rabbit antiserum against purified recombinant HOAS demonstrated that hoas::tnCDC1551 expresses a truncated variant of HOAS (Figure 1A). This was consistent with the distal insertion of Himar-1, which introduced a premature stop codon at nucleotide 3568, resulting in a putative 42 amino acid truncation at the C-terminus of this 1231-residue protein. To explore the essentiality of hoas in Mtb, we introduced a full-length hoas allele into the attL5 site of WTCDC1551 Mtb, generating a merodiploid variant. We replaced native hoas with a hygromycin resistance cassette by allelic exchange via recombineering. By subsequently replacing the attL5 copy of hoas with a vector conferring zeocin resistance, we generated ΔhoasCDC1551. Deletion of the gene was confirmed by Southern blot (Figure S1) and by absence of the protein on immunoblot (Figure 1A). Generation of ΔhoasCDC1551 established that hoas is not essential for in vitro growth of Mtb in standard 7H9 complete medium.
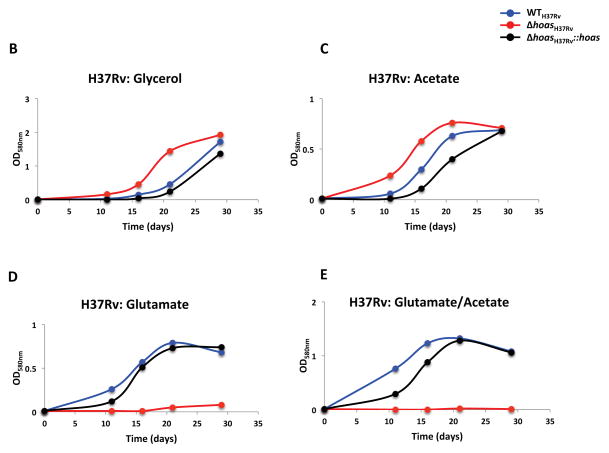
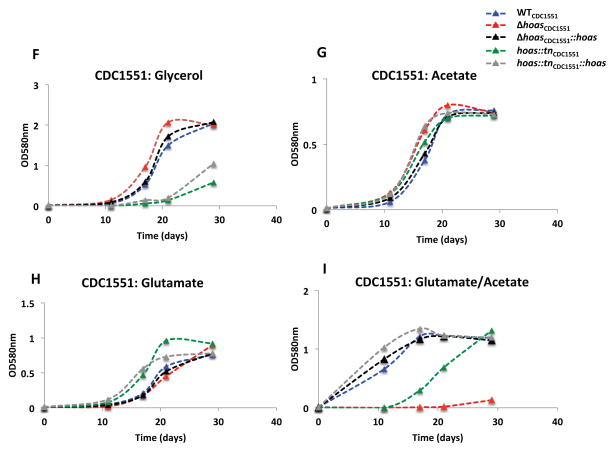
Figure 1. Characterization in vitro of Mtb strains with hoas deleted or disrupted by transposon insertion.
(A) Immunoblot of HOAS in lysates of WTCDC1551, hoas::tnCDC1551, Δ hoasCDC1551, hoas::tnCDC1551::hoas, and Δ hoasCDC1551::hoas using antiserum against HOAS. Antiserum against DlaT was used as a loading control. (B–E) Growth curves of WTH37Rv, ΔhoasH37Rv, and ΔhoasH37Rv::hoas in modified Sauton’s minimal medium supplemented with either (B) glycerol, (C) acetate, (D) glutamate, or (E) glutamate and acetate, each at 0.2%. (F–I) Growth curves of WCDC1551, Δ hoasCDC1551, Δ hoasCDC1551::hoas, hoas::tnCDC1551, and hoas::tnCDC1551::hoas in modified Sauton’s minimal medium supplemented with either (F) glycerol, (G) acetate, (E) glutamate, or (I) glutamate and acetate, each at 0.2%. All results are representative of at least three experiments.
To test if phenotypes of hoas::tnCDC1551 and ΔhoasCDC1551 were due to disruption of hoas, we complemented both strains with the full length hoas allele under the control of a constitutive hsp60 promoter. Complementation restored HOAS protein to WT levels in both mutants (Figure 1A).
3.2 Growth on defined carbon sources
We next compared the in vitro growth profiles of hoas::tnCDC1551, ΔhoasCDC1551, and ΔhoasH37Rv in a modified Sauton’s minimal medium supplemented with a single carbon source or a combination of two carbon sources. Deletion mutants ΔhoasCDC1551 and ΔhoasH37Rv grew no less rapidly than their respective WT parental strains when cultured in either glycerol or acetate (Figure 1B and C, F and G). However, as reported elsewhere [12] and recapitulated here, glutamate, either as sole carbon source or in combination with glycerol or acetate in equal amounts by weight, greatly suppressed growth of ΔhoasH37Rv (Figure 1D and E). In striking contrast, ΔhoasCDC1551 grew as well as WTCDC1551 with glutamate as sole carbon source (Figure 1H). Nonetheless, growth of ΔhoasCDC1551 was abolished when acetate, permissive for growth on its own, was added along with glutamate (Figure 1I). On the other hand, and again in striking contrast to results for ΔhoasH37Rv, glycerol (as opposed to glutamate) stalled hoas::tnCDC1551 growth for approximately 20 days (Figure 1F). Moreover, growth of hoas::tn CDC1551, like growth of ΔhoasCDC1551, was suppressed when glutamate was combined with acetate (Figure 1I). Complementation with full length HOAS in hoas::tnCDC1551, ΔhoasH37Rv, and ΔhoasH37Rv, generating hoas::tnCDC1551::hoas, ΔhoasCDC1551::hoas, and ΔhoasH37Rv::hoas, respectively, rescued all their observed growth defects, with one exception: the suppression of growth of hoas::tnCDC1551::hoas in glycerol (Figure 1F).
3.3 Course of infection in mice
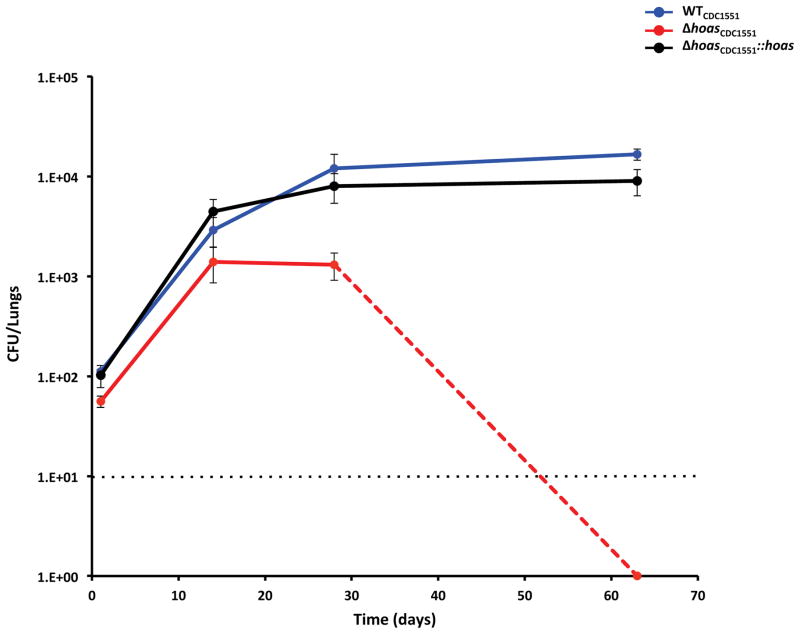
Next, we infected C57BL/6 mice with approximately 100 colony forming units of bacteria per lung of aerosolized WTCDC1551, ΔhoasCDC1551, or ΔhoasCDC1551::hoas. At day 14, ΔhoasCDC1551 grew to a level of CFU in lungs that was about 0.5 log10 units lower than the CFU of WTCDC1551. CFU of ΔhoasCDC1551 rose no further between days 14 and 28 and then declined, so that by day 62, no ΔhoasCDC1551 colonies were recovered from mouse lungs after minimal dilution of the organ homogenate, such that the limit of detection was only 10 CFU (Figure 2). Thus, deletion of hoas in the CDC1551 background severely attenuated Mtb in vivo, and this defect was complemented by the WT allele. As noted elsewhere, ΔhoasH37Rv was attenuated in the mouse as well [12], but not as severely as seen here for ΔhoasCDC1551. Moreover, WTH37Rv established much higher CFU levels than WTCDC1551, for which only about 104 CFU were recovered, similar to results in another study [9].
Figure 2. Phenotype in mice of WT, hoas-deleted and hoas-disrupted Mtb strains on the CDC1551 background.
Bacterial CFU counts in lungs of mice infected with WTCDC1551, Δ hoasCDC1551, or Δ hoasCDC1551::hoas. Data are means ± SEM for five mice per time point (four on day 1). Dashed line indicates the limit of detection. For Δhoas CDC1551 no colonies were recovered at day 62.
3.4 Whole genome re-sequencing
In an effort to understand the discrepancies in the growth phenotypes observed between hoas::tnCDC1551, ΔhoasCDC1551, and ΔhoasH37Rv, and the low bacillary loads of WTCDC1551 in mouse lungs, we re-sequenced the genomes of five strains: WTCDC1551, ΔhoasCDC1551, hoas::tnCDC1551, WTH37Rv and ΔhoasH37Rv (Table 1). WTH37Rv and ΔhoasH37Rv harbored mutations relative to the published H37Rv sequence (NC_000962.3) that were also shared among five other laboratory strains, and, in particular, were most similar to the strain H37RvCO [13]. In terms of novel mutations, both WTH37Rv and ΔhoasH37Rv share a non-synonymous mutation M69L in Rv0516c (possible anti-anti sigma factor). In addition, the WTH37Rv strain had two other unique mutations not found in the deletion mutant. These included a frameshift in residue 1242 of rv2932 (ppsB, component of type I polyketide synthase) and H80Q in Rv0505c (serB1, phosphoserine phosphatase). These were most likely acquired in the parental stock subsequent to generation of the deletion mutant, as H37Rv/pNIT(kan)::RecET-SacB [12] was plated on sucrose to ensure the loss of the recombineering plasmid. The only unique mutation identified in the ΔhoasH37Rv mutant, other than deletion of rv1248c, was a novel insertion of the transposable element IS6110 in rv1358 (supplementary to the 16 known insertion sites in H37Rv). rv1358 is a transcription factor whose role is currently unknown. The growth impairment observed on glutamate for the ΔhoasH37Rv strain, compared to the parental strain (H37Rv), is most likely explained by the deletion of the HOAS gene, because it could be complemented with the WT allele of rv1248c.
Table 1. Results of whole genome re-sequencing.
Identified mutations in WTCDC1551, Δ hoasCDC1551, hoas::tnCDC1551, WTH37Rv and Δ hoasH37Rv that differ from their respective reference strains. Boldface indicates polymorphisms that are unique; non-boldfaced polymorphisms are shared between two or more strains.
| strain | polymorphisms | annotation |
|---|---|---|
|
| ||
| WTCDC1551 | rv2672:R320Q | Possible secreted protease |
| rv3303c: +A (1bp ins) in T410 | LpdA: NAD(P)H quinone reductase | |
|
| ||
| Δ hoasCDC1551 | Δrv1248c | HOAS |
| rv2932: -T (frameshift in aa 420/1538) | PpsB: Pthiocerol synthesis type-I polyketide synthase | |
| rv3919c: G37R | GidB: rRNA methyltransferase | |
| rv2672:R320Q | Possible secreted protease | |
| rv3303c: +A (1bp ins) in T410 | LpdA: NAD(P)H quinone reductase | |
|
| ||
| hoas::tnCDC1551 | rv1248c::Tn (in aa 1189/1242) | HOAS |
| rv0472c:A24A | Probable TetR-family transcription factor | |
| rv1812c:P167A | Probable dehydrogenase | |
| MT1801:V279L | Molybdopterin Oxidoreductase (not present in H37Rv) | |
|
| ||
| WTH37Rv | rv0516c:M69L | Possible anti-anti sigma factor |
| rv0505c:H80Q | SerB1: Phosphoserine phosphatase | |
| rv2932: -C (frameshift in aa 1242/1538) | PpsB: Phthiocerol synthesis type-I polyketide synthase | |
|
| ||
| ΔhoasH37Rv | Δrv1248c | HOAS |
| rv0516c:M69L | Possible anti-anti sigma factor | |
| rv1358:IS6110 insertion | Probable transcription factor | |
Identified mutations in WTCDC1551, Δ hoasCDC1551, hoas::tnCDC1551, WTH37Rv and Δ hoasH37Rv that differ from their respective reference strains. Boldface indicates polymorphisms that are unique; non-boldfaced polymorphisms are shared between two or more strains.
The hoas::tnCDC1551 and ΔhoasCDC1551 mutants each exhibited several unique polymorphisms compared to the parental WTCDC1551 sequence. The transposon-insertion mutant, hoas::tnCDC1551, had unique SNPs in rv0472c (A24A), MT1802 (P167A), and rv1812c (V279L). The mutation in rv0472c is synonymous and hence presumably silent. Rv1812c is annotated as a dehydrogenase but its substrates are unknown. MT1801, annotated as a molybdopterin oxidoreductase, is encoded in a region of the CDC1551 genome that is deleted from H37Rv. It is not apparent how mutations in either enzyme might impart sensitivity to glycerol or avoid the growth inhibitory effect of glutamate, as compared to ΔhoasH37Rv.
As noted, the recombineered deletion mutant, ΔhoasCDC1551, was also able to grow on glutamate (unlike ΔhoasH37Rv), and its growth defect on glutamate plus acetate was only rescued by complementation after a delay that was not observed in ΔhoasH37Rv. However, after filtering out mutations shared between the parental CDC1551 strain and the deletion mutant, and discounting the effect of the frameshift in ppsB (which should only affect PDIM biosynthesis), the only unique mutation left in the ΔhoasCDC1551 mutant was G37R in rv3919c (gidB). The latter SNP is unlikely to explain the growth phenotype, as GidB is an rRNA methyltransferse. The mutation is potentially due to selection on streptomycin during the mutant generation protocol, as mutations in gidB are often found to confer streptomycin resistance [14].
The mutant strain ΔhoasCDC1551 and WTCDC1551 also had several unique polymorphisms not observed in other stocks of CDC1551, such as the transposon insertion mutant hoas::tnCDC1551 (which was generated in a different lab). These include a SNP in Rv2672 (possible secreted protease) and a frameshift in LpdA. It is possible that these SNPs are related to the relative attenuation of the WTCDC1551 and ΔhoasCDC1551 strains in mice, compared to the bacillary loads typically achieved by H37Rv-derived strains. However, since these SNPs are shared by ΔhoasCDC1551 and WTCDC1551, they should not have any bearing on the in vitro growth phenotype of ΔhoasCDC1551. The genome sequence we determined for WTCDC1551 had hundreds of differences from the published CDC1551 reference sequence, NC_002755.2, which are assumed to be due to corrections in original sequencing errors, including many ambiguous nucleotides and short 1 bp indels.
4. Discussion
We began our studies of the biology of HOAS by taking advantage of the availability of the transposon mutant in the CDC1551 background. We completed our in vitro studies of hoas::tnCDC1551, hoas::tnCDC1551::hoas, ΔhoasCDC1551 and ΔhoasCDC1551::hoas before testing the phenotype of WTCDC1551 and ΔhoasCDC1551 in the mouse. The marked attenuation of WTCDC1551 in the mouse was unanticipated. That observation led us to conclude that a study of the biology of HOAS in intact Mtb in vitro might be compromised in the CDC1551 background. We therefore recapitulated our studies in the WTH37Rv background after generating ΔhoasH37Rv and ΔhoasH37Rv::hoas [12]. This gave us the opportunity to compare and contrast the phenotypic consequences of identical gene deletions in two different Mtb strains –ΔhoasCDC1551 and ΔhoasH37Rv – that were engineered with the same genetic tools. We believe that the discrepancies we observed, while unexplained, have cautionary value.
First, we observed a startling difference in growth profiles for the deletion mutants in glutamate: glutamate was suppressive for ΔhoasH37Rv, but supportive for ΔhoasCDC1551. Glutamate also abolished growth of ΔhoasH37Rv when added to acetate. For ΔhoasCDC1551, the combination of glutamate and acetate was growth suppressive, but as noted, glutamate alone supported growth.
Second, growth of hoas::tnCDC1551, a strain expressing a truncated version of HOAS, was defective in glycerol (as opposed to glutamate) and this defect was not rescued by complementation. This result was starkly different from what was observed with both deletion mutants, whose phenotypes were all complemented by the wild type allele.
Third, in mixtures of glutamate and acetate, hoas::tnCDC1551 and ΔhoasCDC1551 had opposing phenotypes, yet in each case these particular phenotypes were normalized by genetic complementation. The genome sequences of WTH37Rv and WTCDC1551 have a considerable number of differences, given that strains H37Rv and CDC1551 are derived from different TB lineages (i.e. T clade versus X clade, respectively [15, 16]), including approximately 1300 SNPs between them, as well as several inserted/deleted genes and genomic regions. Thus there are many potential reasons why there might be differences in phenotypes between mutants derived from different genetic backgrounds.
In other respects, both deletion mutants exhibited similar properties. As reported elsewhere, ΔhoasH37Rv was much more susceptible to reactive nitrogen intermediates in vitro than WTH37Rv [12]. Likewise, ΔhoasCDC1551 was far more susceptible to reactive nitrogen intermediates in vitro than WTCDC1551 (data not shown). Exposure to glutamate induced selective increases in intracellular α-ketoglutarate and succinic semialdehyde levels within ΔhoasH37Rv [12]. Levels of these same metabolites were also selectively and extensively elevated when ΔhoasCDC1551 was grown in the presence of glutamate, despite this strain’s lack of a growth defect with glutamate as sole carbon source (data not shown). Finally, both deletion mutants were attenuated in mice, although attenuation was more severe on the CDC1551 background.
In an attempt to clarify phenotypic discrepancies between both deletion mutants and between them and hoas::tnCDC1551, as well as to understand why WTCDC1551 was so attenuated on its own, we sequenced the genomes of the three mutant strains and their WT parents. We discovered that both ΔhoasCDC1551 and WTH37Rv genomes contained deletions in rv2932, the gene encoding the phthiocerol synthesis type-I polyketide synthase, PpsB, in the ppsA-E operon that encodes enzymes vital for synthesis of phthiocerol dimycocerosate (PDIM). PDIM is a wax in the cell wall necessary for full virulence of Mtb strains in mice, and is frequently lost during in vitro passage [17, 18]. This polymorphism most likely contributed to the slightly reduced CFU count we observed for WTH37Rv in mouse lungs compared to other reports using WTH37Rv [12]. However, PDIM biosynthesis was not genetically disrupted in WTCDC1551, whose sequence hardly differed from that of WTCDC1551 used in other laboratories. Thus PDIM deficiency did not seem likely to account for our fourth surprising observation, the marked attenuation of WTCDC1551.
Contrary to our expectations, whole genome re-sequencing results did not appear to explain the sole phenotypic discrepancy observed in vitro (growth in glutamate) between the two hoas deletion strains. The deletion of HOAS in ΔhoasH37Rv explains the sensitivity to glutamate in an H37Rv background [12]. Relative to this, the ability of ΔhoasCDC1551 to grow on glutamate was unexpected; however, the sole unique secondary mutation observed in ΔhoasCDC1551 (compared to the parental CDC1551) was unable to explain this anomalous phenotype. Nor did any one of the unique polymorphisms seem likely to explain the phenotypic disparities among ΔhoasCDC1551, ΔhoasCDC1551, and hoas::tnCDC1551. The hoas::tn CDC1551 genome harbors only three unique mutations compared to ΔhoasCDC1551, despite its unique growth profile, which suggests caution in interpreting further results with this transposon-insertion strain.
Our observations appear to challenge the underlying premise of genetic complementation: that restoration of WT behavior upon supplying the WT allele establishes that a mutant’s phenotype is due specifically to disruption or deletion of the corresponding gene. Our observations also illustrate that re-sequencing of the genome of a mutant may not always reveal potential explanations for its phenotype. Among speculative explanations for these observations we suggest the following. (i) The observed polymorphisms, acting in concert, may have epistatic effects that would be unanticipated from the annotated function of each polymorphic gene considered separately. (ii) There may be polymorphisms in regions of the genome that were not well covered by sequencing. (iii) There could be copy-number variation in tandem-repeat sequences (e.g. MIRU sequences), which are difficult to resolve with short-read sequencing data. (iv) Single nucleotide polymorphisms that are silent in the annotated coding region may change sense in an unannotated open reading frame or change function in an unannotated intergenic regulatory sequence on the opposite strand. (v) RNA editing may occur in Mtb, so that the sequence of some transcripts may differ from the sequence encoded in the DNA. (vi) Epigenetic changes, for example differences in DNA methylation patterns, may affect gene expression in Mtb, leading to different growth phenotypes [19].
5. Conclusions
This study illustrates three findings that reveal limitations in what is currently the standard approach to mycobacterial genetics: (i) a transposon mutant’s growth profile differed from that of a deletion mutant in the same strain background, and both could be complemented; (ii) the same gene deletion in two genetically similar strain backgrounds gave different phenotypes, though each could be complemented; and (iii) whole genome re-sequencing did not provide an obvious explanation for (i) or (ii).
Supplementary Material
Southern blot design described in [12]. (A) Southern blot of digested Mtb DNA from ΔhoasCDC1551 (Lane 1), ΔhoasCDC1551 candidates (ΔhoasCDC::pGMCS-hsp60hoas) before replacement transformation (Lane 2), and WTCDC1551. Bands are at expected sizes.
Table S1. Sequencing Statistics.
Acknowledgments
We thank Dirk Schnappinger (Weill Cornell Medical College) for plasmids and advice, Kristin Burns-Huang (Weill Cornell Medical College) for advice, and Xiuju Jiang (Weill Medical College) for assistance. This work was supported by grant RO1 AI064768, by the Milstein Program in Translational Medicine and by Bill and Melinda Gates Foundation grant OPP1024055 to J. Sacchettini. The Department of Microbiology and Immunology at Weill Cornell Medical College is supported by the William Randolph Hearst Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sassetti CM, Rubin EJ. Genetic requirements for mycobacterial survival during infection. Proc Natl Acad Sci U S A. 2003;100:12989–12994. doi: 10.1073/pnas.2134250100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lamichhane G, Bishai W. Defining the ‘survivasome’ of Mycobacterium tuberculosis. Nat Med. 2007;13:280–282. doi: 10.1038/nm0307-280. [DOI] [PubMed] [Google Scholar]
- 3.Sassetti CM, Boyd DH, Rubin EJ. Genes required for mycobacterial growth defined by high density mutagenesis. Mol Microbiol. 2003;48:77–84. doi: 10.1046/j.1365-2958.2003.03425.x. [DOI] [PubMed] [Google Scholar]
- 4.Griffin JE, Gawronski JD, Dejesus MA, Ioerger TR, Akerley BJ, Sassetti CM. High-resolution phenotypic profiling defines genes essential for mycobacterial growth and cholesterol catabolism. PLoS Pathog. 2011;7:e1002251. doi: 10.1371/journal.ppat.1002251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang YJ, Ioerger TR, Huttenhower C, Long JE, Sassetti CM, Sacchettini JC, Rubin EJ. Global assessment of genomic regions required for growth in Mycobacterium tuberculosis. PLoS Pathog. 2012;8:e1002946. doi: 10.1371/journal.ppat.1002946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Balakrishnan A, Jordan F, Nathan CF. Influence of allosteric regulators on individual steps in the reaction catalyzed by Mycobacterium tuberculosis 2-hydroxy-3-oxoadipate synthase. J Biol Chem. 2013;288:21688–21702. doi: 10.1074/jbc.M113.465419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lamichhane G, Zignol M, Blades NJ, Geiman DE, Dougherty A, Grosset J, Broman KW, Bishai WR. A postgenomic method for predicting essential genes at subsaturation levels of mutagenesis: application to Mycobacterium tuberculosis. Proc Natl Acad Sci U S A. 2003;100:7213–7218. doi: 10.1073/pnas.1231432100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Valway SE, Sanchez MP, Shinnick TF, Orme I, Agerton T, Hoy D, Jones JS, Westmoreland H, Onorato IM. An outbreak involving extensive transmission of a virulent strain of Mycobacterium tuberculosis. N Engl J Med. 1998;338:633–639. doi: 10.1056/NEJM199803053381001. [DOI] [PubMed] [Google Scholar]
- 9.Barczak AK, Domenech P, Boshoff HI, Reed MB, Manca C, Kaplan G, Barry CE. In vivo phenotypic dominance in mouse mixed infections with Mycobacterium tuberculosis clinical isolates. J Infect Dis. 2005;192:600–606. doi: 10.1086/432006. [DOI] [PubMed] [Google Scholar]
- 10.Manca C, Tsenova L, Barry CE, Bergtold A, Freeman S, Haslett PA, Musser JM, Freedman VH, Kaplan G. Mycobacterium tuberculosis CDC1551 induces a more vigorous host response in vivo and in vitro, but is not more virulent than other clinical isolates. J Immunol. 1999;162:6740–6746. [PubMed] [Google Scholar]
- 11.North RJ, Ryan L, LaCource R, Mogues T, Goodrich ME. Growth rate of mycobacteria in mice as an unreliable indicator of mycobacterial virulence. Infect Immun. 1999;67:5483–5485. doi: 10.1128/iai.67.10.5483-5485.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maksymiuk C, Balakrishnan A, Bryk R, Rhee K, Nathan C. E1 of alpha-ketoglutarate dehydrogenase defends Mycobacterium tuberculosis against glutamate anaplerosis and nitroxidative stress. Proc Natl Acad Sci USA. 2015 doi: 10.1073/pnas.1510932112. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ioerger TR, Feng Y, Ganesula K, Chen X, Dobos KM, Fortune S, Jacobs WR, Mizrahi V, Parish T, Rubin E, Sassetti C, Sacchettini JC. Variation among genome sequences of H37Rv strains of Mycobacterium tuberculosis from multiple laboratories. J Bacteriol. 2010;192:3645–3653. doi: 10.1128/JB.00166-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wong SY, Lee JS, Kwak HK, Via LE, Boshoff HI, Barry CE. Mutations in gidB confer low-level streptomycin resistance in Mycobacterium tuberculosis. Antimicrob Agents Chemother. 2011;55:2515–2522. doi: 10.1128/AAC.01814-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sarkar R, Lenders L, Wilkinson KA, Wilkinson RJ, Nicol MP. Modern lineages of Mycobacterium tuberculosis exhibit lineage-specific patterns of growth and cytokine induction in human monocyte-derived macrophages. PLoS One. 2011;7:e43170. doi: 10.1371/journal.pone.0043170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brudey K, et al. Mycobacterium tuberculosis complex genetic diversity: mining the fourth international spoligotyping database (SpolDB4) for classification, population genetics and epidemiology. BMC Microbiol. 2006;6:23. doi: 10.1186/1471-2180-6-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cox JS, Chen B, McNeil M, Jacobs WR. Complex lipid determines tissue-specific replication of Mycobacterium tuberculosis in mice. Nature. 1999;402:79–83. doi: 10.1038/47042. [DOI] [PubMed] [Google Scholar]
- 18.Domenech P, Reed MB. Rapid and spontaneous loss of phthiocerol dimycocerosate (PDIM) from Mycobacterium tuberculosis grown in vitro: implications for virulence studies. Microbiology. 2009;155:3532–3543. doi: 10.1099/mic.0.029199-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shell SS, Prestwich EG, Bake S-H, Shah RR, Sassetti CM, Dedon PC, Fortune SM. DNA methylation impacts gene expression and ensures hypoxic survival of Mycobacterium tuberculosis. PLoS Pathogens. 2013;9:e1003419. doi: 10.1371/journal.ppat.1003419. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Southern blot design described in [12]. (A) Southern blot of digested Mtb DNA from ΔhoasCDC1551 (Lane 1), ΔhoasCDC1551 candidates (ΔhoasCDC::pGMCS-hsp60hoas) before replacement transformation (Lane 2), and WTCDC1551. Bands are at expected sizes.
Table S1. Sequencing Statistics.