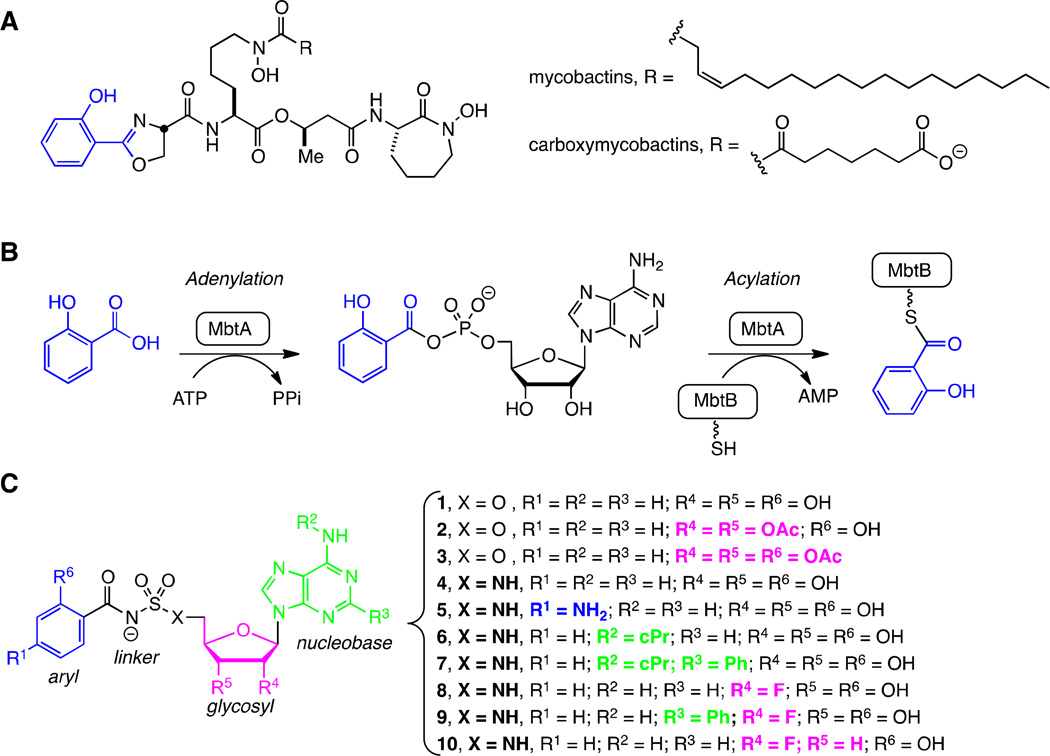
Figure 1.
Mycobactins, biosynthesis, and inhibitors. A. Structures of the lipid soluble mycobactins and water soluble mycobactins (refered to as carboxymycobactins) produced by Mtb. The mycobactins and carboxymycobactins are synthesized as a suite of compounds that differ in the chain length of the lipid tail on the central lysine residue. The most abundant chain length is depicted. B. Biosynthesis of all mycobactins is initiated with the ATP-dependent activation of salicylic acid by MbtA to form an intermediate acyl-adenylate. In a second half-reaction, MbtA ligates salicylic acid onto the N-terminal aryl acid carrier protein domain of MbtB. MbtB, in combination with MbtE, MbtC, MbtD, and MbtF (not shown), sequentially builds the mycobactin peptidic core scaffold. C. 5′-O-[N-(salicyl)sulfamoyl]adenosine (1) was the first described inhibitor of MbtA and is considered a bisubstrate inhibitor that mimics the intermediate acyl-adenylate. This modular inhibitor scaffold contains aryl (blue), linker (black), glycosyl (pink), and nucleobase (green) domains. Prior SAR studies have examined the importance of each atom of this scaffold and provided a comprehensive understanding of the structure-activity relationships (SAR) that govern enzyme inhibition and whole-cell activity. This information has been used to design prodrugs 2–3 and analogs 4–10 in order to explore the SAR that governs pharmacokinetic behavior.