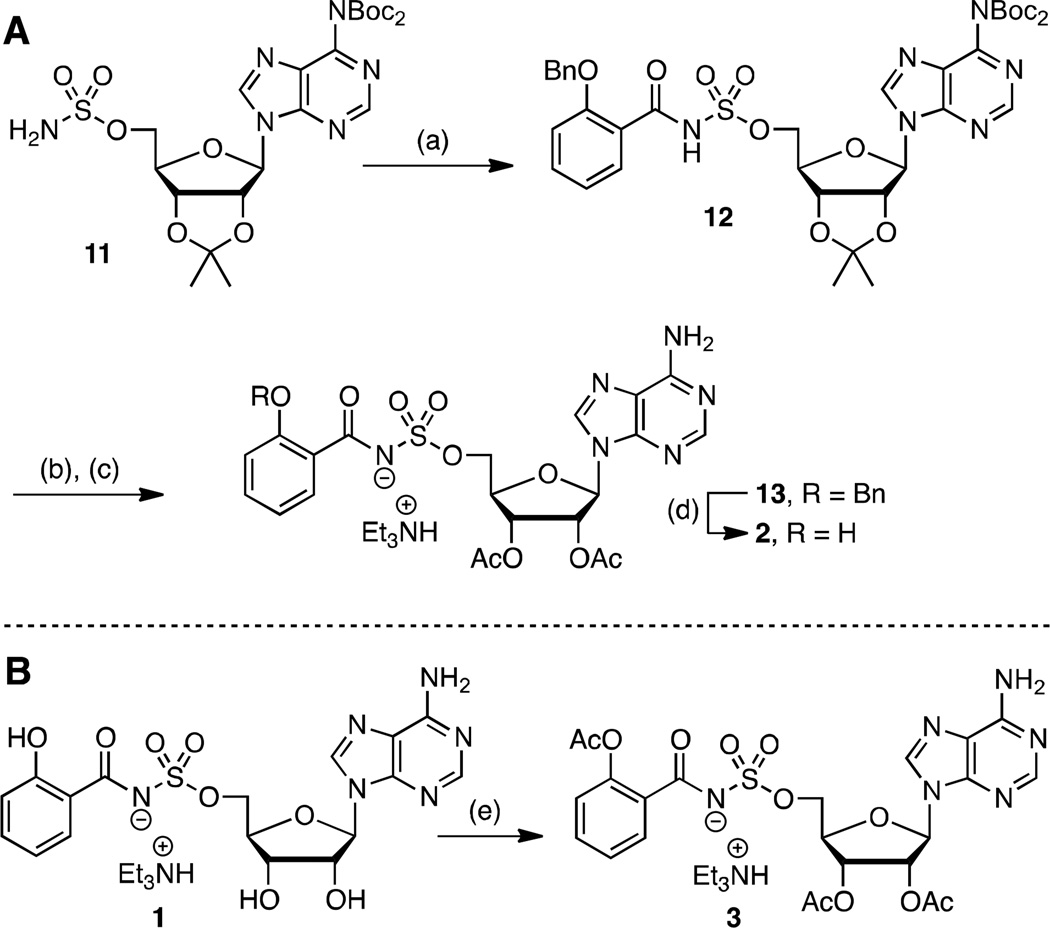
Scheme 1. a. Synthesis of prodrugs 2 and 3.
aReaction conditions: (a) N-hydroxysuccinimidyl 2-benzyloxybenzoate, Cs2CO3, DMF, 0 °C to rt, 21 h, 62%; (b) 80% aqueous TFA, 0 °C to rt, 4 h; (c) Ac2O, pyridine, DMF, rt, 16 h, 63% (2 steps); (d) H2, Pd/C, MeOH, rt, 2 h, 42%; (e) Ac2O, pyridine, DMF, rt, 20 h, 46%.