Scheme 1.
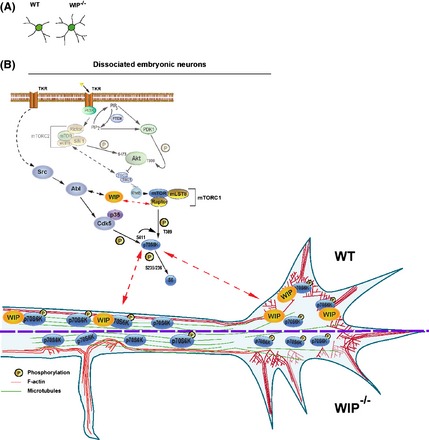
WIP controls neuritic branching through the modulation of the activity level of the Abl‐mTORC1‐S6K pathway. (A) Archetypical morphology of WT and WIP −/− neurons at 24 h post plating derived from our quantitative IF and biochemical data. (B) Our data permit to postulate that in a model system of dissociated embryonic neurons, WIP determines the level of neuritic branching via the positive regulation of the mTORC1‐S6K pathway activity for the initial stages of neuronal development. The effect in two phospho‐epitopes of S6K allows hypothesizing that, in addition to mTORC1 activation (S6K‐pThr389), Abl may be implicated in this initial regulation through Cdk5‐p35 kinase complex (S6K‐pSer411). As the levels of active S6K and F‐actin are equivalent in the growth cones of both genotypes, WIP might play a potential role as a scaffold protein tethering S6K‐pThr389 to MF in actin‐rich areas. Moreover, WIP −/− neurons present reduced levels of S6K‐pThr389 over the neurites and increased density of neuritic filopodia compared to controls. These data lead us to propose that WIP and active S6K may act as negative regulators of the F‐actin protrusive activity along the neuritic shaft.
