Abstract
Bacterial biofilm infections remain prevalent reasons for implant failure. Dental implant placement occurs in the oral environment, which harbors a plethora of biofilm-forming bacteria. Due to its trans-mucosal placement, part of the implant structure is exposed to oral cavity and there is no effective measure to prevent bacterial attachment to implant materials. Here, we demonstrated that UV treatment of titanium immediately prior to use (photofunctionalization) affects the ability of human polymicrobial oral biofilm communities to colonize in the presence of salivary and blood components. UV-treatment of machined titanium transformed the surface from hydrophobic to superhydrophilic. UV-treated surfaces exhibited a significant reduction in bacterial attachment as well as subsequent biofilm formation compared to untreated ones, even though overall bacterial viability was not affected. The function of reducing bacterial colonization was maintained on UV-treated titanium that had been stored in a liquid environment before use. Denaturing gradient gel-electrophoresis (DGGE) and DNA sequencing analyses revealed that while bacterial community profiles appeared different between UV-treated and untreated titanium in the initial attachment phase, this difference vanished as biofilm formation progressed. Our findings confirm that UV-photofunctionalization of titanium has a strong potential to improve outcome of implant placement by creating and maintaining antimicrobial surfaces.
Keywords: Photofunctionalization, titanium, bacteria, biofilm, peri-implantitis
1. Introduction
Dental implants have become a popular restorative choice with an initial success rate of up to 98% [1]. Depending on implant type, this success rate declines over time and ranges between 90.1 and 95.4% after 5 years, with a further reduction to about 89% and 83% after 10 and 16 years, respectively – the longest observation period reported so far [2]. Older patients, those with systemic conditions, smoking status [3, 4] or prior periodontal disease [5–7] are affected by an overall higher failure rate. Most complications can be attributed to lack of sufficient osseointegration and infection. Hence, complete and infection-free establishment of bone-implant integration has become a persistent challenge in oral rehabilitation. The major causes for implant-related infections and inflammatory responses are microbial biofilms, which can form on all currently employed implant materials [8–10]. Biofilm formation is a multi-step process that starts with the bacterial attachment to natural or artificial surfaces. This initial interaction between bacteria and surface can occur directly via charged groups (e.g. phosphoryl-, carboxyl-, and amino-groups) present on their complex cell surface layer [11]. Since the bacterial cell surface is in direct contact with the environment, their charged cell surface layer groups are able to interact with ions or charged molecules present on the implant material surfaces [12]. In addition to this direct interaction, microorganisms can exploit other molecules including host proteins that adhere to the implant material to achieve surface colonization [13]. In the oral environment relevant for dental implant dentistry, molecules derived from saliva such as the proteins involved in pellicle formation that provide additional bacterial adhesion sites as well as blood components, can attach to the implant material and change certain surface characteristics [14–16]. Therefore, implant surface characteristics and the molecules from relevant bodily fluids that can attach to the implant material are important determinants in the amount and composition of bacterial biofilm to be formed.
Recent approaches to address the challenge of implant failure include ultraviolet (UV)-mediated photofunctionalization of titanium (Ti) [17–19], a popular implant material due to its excellent biocompatibility, corrosion resistance and its ability to promote osseointegration [20–23]. UV irradiation leads to the modification of titanium implant surfaces from a hydrophobic to a superhydrophilic state and removes hydrocarbon contamination [17, 24, 25]. These extreme changes in surface properties have been studied extensively for their effect on enhancing osteoblast attachment and proliferation, which leads to greatly improved osseointegration of titanium implants [26–29]. Despite this very encouraging extensive research regarding bone-implant integration, very little is known to date regarding the effect of photofuntionalization on bacterial attachment and biofilm formation on titanium surfaces despite its importance for lasting implant success [30]. A recent report demonstrated that UV treatment of Ti surfaces can reduce attachment and monospecies biofilm formation of Staphylococcus aureus and Streptococcus pyogenes [30], the major pathogens for orthopedic implant infections. While this is a very promising observation, dental implants are exposed to a more challenging environment: the microbiota of the oral cavity. Extensive 16S rRNA gene sequencing and microbiome studies revealed that over 600 different oral microbial taxa colonize the various surfaces present in the mouth [31]. The bacterial species implicated in dental implant-associated diseases such as peri-mucositis and peri-implantitis are generally very similar to those associated with periodontal diseases [32, 33]. Many of these bacteria are able to readily attach to surfaces including titanium implants and employ saliva and/or blood-derived proteins for enhanced attachment [16, 34]. This ability to exploit host fluids for attachment presents an additional challenge in biofilm prevention, especially during implant placement, when the sterile surface becomes exposed to the oral environment and the surgical wound site.
In this study, we investigated if UV-treatment of titanium surfaces has an effect on the attachment and biofilm formation of complex oral microbial communities during time periods that are relevant for the initial implant placement and wound healing directly post-surgery. UV-irradiation-induced titanium surface properties were evaluated and bacterial biomass accumulation at different time points reflecting initial attachment and early biofilm formation events were determined in the presence of salivary and blood components. Community profiles of the attached microorganisms were compared between UV-treated and untreated titanium surfaces.
2. Materials and methods
2.1 Titanium disc preparation, surface analysis and UV treatment
Titanium (Ti) discs (20 mm in diameter and 1.5 mm in thickness) were prepared by machining commercially pure titanium (Grade 2). Titanium disks were autoclaved and stored in the dark for 4 weeks to standardize the age of the titanium, since titanium age is known to affect its biological and osteoconductive capabilities [35, 36]. Titanium disks were treated with UV light for 12 minutes with a photo device (TheraBeam Super Osseo, Ushio Inc., Tokyo, Japan) immediately prior to use [37, 38], while control discs were left untreated. The surface morphology of the discs was examined using scanning electron microscopy (XL30, Philips, Eindhoven, Netherlands) [26]. The hydrophilic and hydrophobic properties of the titanium discs were evaluated by measuring the contact angle of 10 µl ddH2O [19].
2.2 Oral microbial community and culture conditions
We used a previously described cultivable microbial community representative of the complex oral microbiome as model system for bacterial attachment and biofilm formation [39, 40]. The microbial community was grown anaerobically (80% N2, 10% H2, and 10% CO2) at 37°C in a modified rich medium (SHI-FSMS) developed to support a high number of oral taxa from human saliva samples (50% SHI medium [40], 25% filtered saliva, 0.5% mannose, 0.5% sucrose). Initial attachment of cells and biofilm formation were evaluated after 3 and 16 hours incubation, respectively. Overnight oral microbial community culture was adjusted in fresh SHI-FSMS medium to an optical density (OD) at 600 nm of 1, for evaluation of attachment and to an OD600nm of 0.1 for measurement of biofilm formation. For both types of experiments, one ml of the oral microbial community at the respective concentration was placed onto titanium discs immediately after UV-photofunctionalization or directly onto untreated discs in sterilized 12-well polystyrene culture plates (Fisher Scientific). Oral microbial community cultures at the two relevant concentrations used in this study were also inoculated directly into the polystyrene plate to serve as positive controls for bacterial attachment and biofilm formation. Additionally, UV-treated and untreated titanium discs as well as wells without discs were incubated with sterile medium to serve as background controls. Samples were statically incubated at 37°C under anaerobic conditions for 3 or 16 hours corresponding to the respective experiments. To evaluate the continuous effect of UV-treatment, UV-treated and untreated titanium discs were immersed in fresh SHI-FSMS medium for 24 hours at 37°C, under anaerobic conditions prior to inoculation of bacteria. After this pre-incubation period, the medium was removed and overnight oral community culture diluted to an OD600nm of 0.1 was added to disc for analysis of biofilm formation after 16 hours incubation. Concurrently, the diluted community culture was placed on UV-treated and untreated titanium discs surfaces that had not undergone pre-immersion in medium for comparison. For all experiments, medium was removed at the end of the incubation period; the discs were gently transferred into 6-well polystyrene culture plates and washed three times with 5 ml sterile phosphate-buffered saline (PBS) prior to further processing.
2.3 Crystal violet assay
A 0.5% crystal violet solution was used to determine biomass accumulation onto the titanium discs surface and control wells. The PBS-washed titanium discs were placed into a 12-well plate, submerged in one ml crystal violet solution and incubated at room temperature for 20 min. The discs were then carefully transferred to a 6-well plate and washed four times with 5 ml PBS to remove excess crystal violet. The plates were gently shaken for 5 minutes during the last two PBS washes to ensure complete removal of residual dye. After the final PBS wash, the discs were transferred to a new 12-well plate. One ml of 95% ethanol was added and the plate was incubated at room temperature on a rotatory shaker (VWR rocking double platform shaker model 200) at 250 rpm for 15 minutes. The ethanol solution containing the crystal violet stain retained by the biofilms was transferred into 1.5 ml cuvettes (USA Scientific) and the optical density at 595 nm was determined for total biomass evaluation. All experiments were performed in triplicate for each time point and repeated three times to ensure technical and biological reproducibility with the exception of the experiments, in which untreated and UV-photofunctionalized titanium discs were pre-incubated in SHI-FSMS medium for 24 hours prior to bacterial inoculation. These were only repeated twice as technical triplicates.
2.4 Sample Preparation, Confocal Laser Scanning Microscopy, Viability Staining and ImageJ analysis
Samples for assessment of bacterial attachment and biofilm formation on untreated and UV-treated titanium disc surfaces were rinsed three times with PBS to remove unattached bacteria prior to assessing bacterial viability via fluorescent labeling with the LIVE/DEAD BacLightTM Bacterial Viability staining kit (Invitrogen, Carlsbad, CA) according to manufacturer’s instructions. The titanium discs were placed onto a glass cover slip, 35×50 mm (Fisher Scientific) into a 10 µl drop of PBS with the biofilm side facing the objective. Samples were visualized with a PASCAL LSM5 confocal laser-scanning microscope (Zeiss, Jena, Germany). The scanning module of the system was mounted on an inverted microscope (Axiovert 200 M) and samples were imaged through 10x dry (Plan NeoFluar NA 0.3 air) and 40x oil-immersion (Plan NeoFluar NA 1.3 oil) objectives. Excitation wavelengths of 488 nm (Ar laser) and 543 nm (HeNe laser) in combination with 505 to 530 nm bandpass and 560 nm longpass filters, respectively, were employed to reveal live dead distribution of bacterial cells as well as the accumulated biomass.
Image analysis was performed with ImageJ 1.48 [National Institutes of Health (NIH), Bethesda, MD, USA; freeware from http://imagej.nih.gov/ij/download.html]. To measure the area occupied by bacteria, representative confocal images were exported and converted into 16bit. A median filter with a radius of 2 pixels was applied to reduce noise and an intensity threshold was applied to separate the fluorescent bacteria from the background. Next, the image was converted into a binary image and using the ‘analyze particles’ function, all the groups of cells bacteria with a minimal surface area of 2 µm2 were calculated. Analysis of biofilm density was measured by conversion of confocal images into RGB color. First, background subtraction was applied using a sequentially decreasing rolling-ball radius to ensure maximum removal of background noise. Then, the image was assembled into color channels and the integrated density of pixels was calculated. All experiments were performed in technical duplicates for each time point with two biological repetitions.
2.6 DNA Extraction, PCR and denaturing gradient gel-electrophoresis (DGGE)
Microbial cells were harvested from the titanium discs by scraping with a sterile pipette tip and placed into 1.5 ml Eppendorf Tube containing 150 µl PBS. Total genomic DNA was isolated using the MasterPure™ DNA purification kit (EPICENTRE). The concentration of bacterial DNA was determined with a Nanodrop 2000 (Thermo Scientific). Primers Bac1 with a GC clamp (5’-CGC CCG CCG CGC CCC GCG CCC GTC CCG CCG CCC CCG CCC GAC TAC GTG CCA GCA GCC-3’) and Bac2 (5’-GGA CTA CCA GGG TAT CTA ATC C-3’) were used to amplify a region approximately 300-base-pair in length (bp) of the 16S ribosomal RNA gene [41]. PCR amplification was confirmed by electrophoresis in a 1.0% agarose gel. Polyacrylamide gels (8%) were prepared with a denaturing urea/formamide gradient ranging from 40% to 60%. Approximately 45 µl of the PCR product were loaded into each well. The gel was submerged in 1X TAE buffer (40 mM Tris base, 40 mM glacial acetic acid, 1 mM ethylenediaminetetraacetic acid) and the PCR products were separated by electrophoresis for 17 hours at 58°C using a fixed voltage of 60 V in a Bio-Rad DCode System (Bio-Rad Laboratories, Inc., Hercules, CA). Gels were stained with ethidium bromide to visualize the bands on the gel. Gel images were taken with the Molecular Imager Gel Documentation system (Bio-Rad Laboratories). All experiments were performed in triplicate for each time point.
2.7 DNA Sequencing of excised DGGE bands
Bands of interest were excised from the DGGE gel with a sterile razor blade, placed into 1.5-mL tubes containing 15 µl sterile Milli-Q water. The tubes were incubated overnight at 4°C to allow the DNA to diffuse into the water. Five µl of the DNA sample were used as template for re-amplification with the universal primers, Bac1 and Bac2, and the product was sent to Laragen Sequencing & Genotyping (Culver City, CA, USA) for sequencing. For identification, the 16S rDNA sequences were compared with the Human Oral Microbiome Database (HOMD) using BLAST.
2.8 Statistical data analysis
All data are presented as a mean ± standard deviation. Statistical comparisons were performed using the unpaired t-test, one tailed, except for evaluation of the effect of UV treatment on titanium disc surfaces immersed for 24 hours in SHI-FSMS medium prior to bacterial inoculation compared to discs that did not undergo the preimmersion step. In this case, one way analysis of variance (ANOVA) was employed with a Tukey’s posthoc test using GraphPad Prism version 5.0c; p< 0.05 was considered statistically significant.
3. Results
3.1 UV-photofunctionalization increased the hydrophilicity of titanium surfaces without affecting its topography
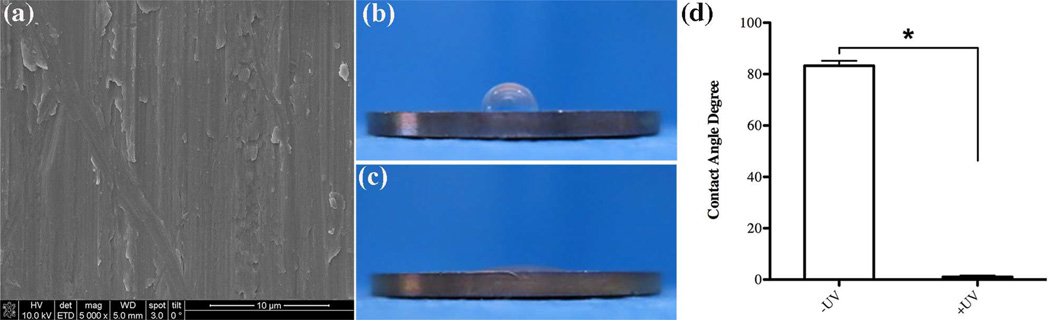
SEM analysis confirmed that after a 12 minute UV-photofunctionalization treatment, the titanium discs used in this study maintained meso-scale parallel traces typical for machine-turned titanium surfaces (Fig. 1a). Hydrophilicity testing demonstrated that UV-treatment resulted in a drastic change in titanium wettability to ddH2O. Untreated control titanium discs were highly hydrophobic with the 10 µl droplet of ddH2O remaining in a semispherical form without spreading and a contact angle higher than 80° (Fig. 1b, d). In contrast, after UV-treatment the titanium discs became very hydrophilic, as evident by the immediate spreading of the ddH2O droplet and a contact angle of less than 5° (Fig. 1c, d).
Fig. 1.
Surface characterization of machined titanium. (a) scanning electron microscopy of UV-treated discs showing surface topography at a 5000x magnification, evaluation of hydrophilicity by contact angle measurement of 10µl ddH2O (b) before and (c) after UV-treatment on the Ti discs and (d) statistical significance testing of contact angle values for -UV and +UV titanium discs. Statistically significant differences are indicated as: * p<0.0001. Data represent the mean ± SD of triplicate data of one independent experiment.
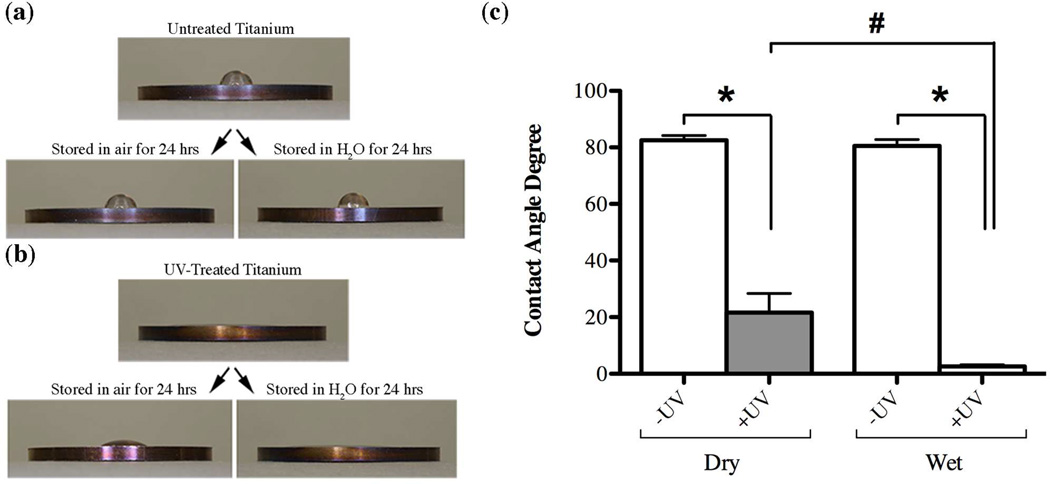
The effect of UV-treatment on surface characteristics was maintained for more than 24 hours (Fig. 2). While the untreated control discs remained hydrophobic regardless if they were stored in air or in a liquid environment (Fig. 2a, c), UV-treated discs retained their hydrophilic characteristics especially when the discs were kept in a liquid environment (Fig. 2b, c). Even though UV-treated titanium surfaces were still relatively hydrophilic after 24 hours of storage in air compared to their untreated counterparts, surface wettability reversed significantly towards more hydrophobic during the experimental time period (Fig. 2b, c). This difference in contact angle between UV-treated discs subjected to different storage conditions was statistically significant (p<0.0001).
Fig. 2.
Measurement of the contact angle of 10µl ddH2O to evaluate changes in hydrophilicity of titanium discs surfaces (a) without and (b) after UV-photofunctionalization treatment as well as after subsequent storage in air or a liquid environment for 24 hours. Comparison of contact angles values (c) between untreated (white bars, - UV) and UV-treated (gray bars, +UV) after storage in air (Dry) or a liquid environment (Wet). Statistically significant differences are indicated as: * p<0.0001, # p=0.0042. Data represent the mean ± SD of triplicate data from one independent experiment.
3.2 UV-treatment reduced bacterial attachment on titanium surfaces
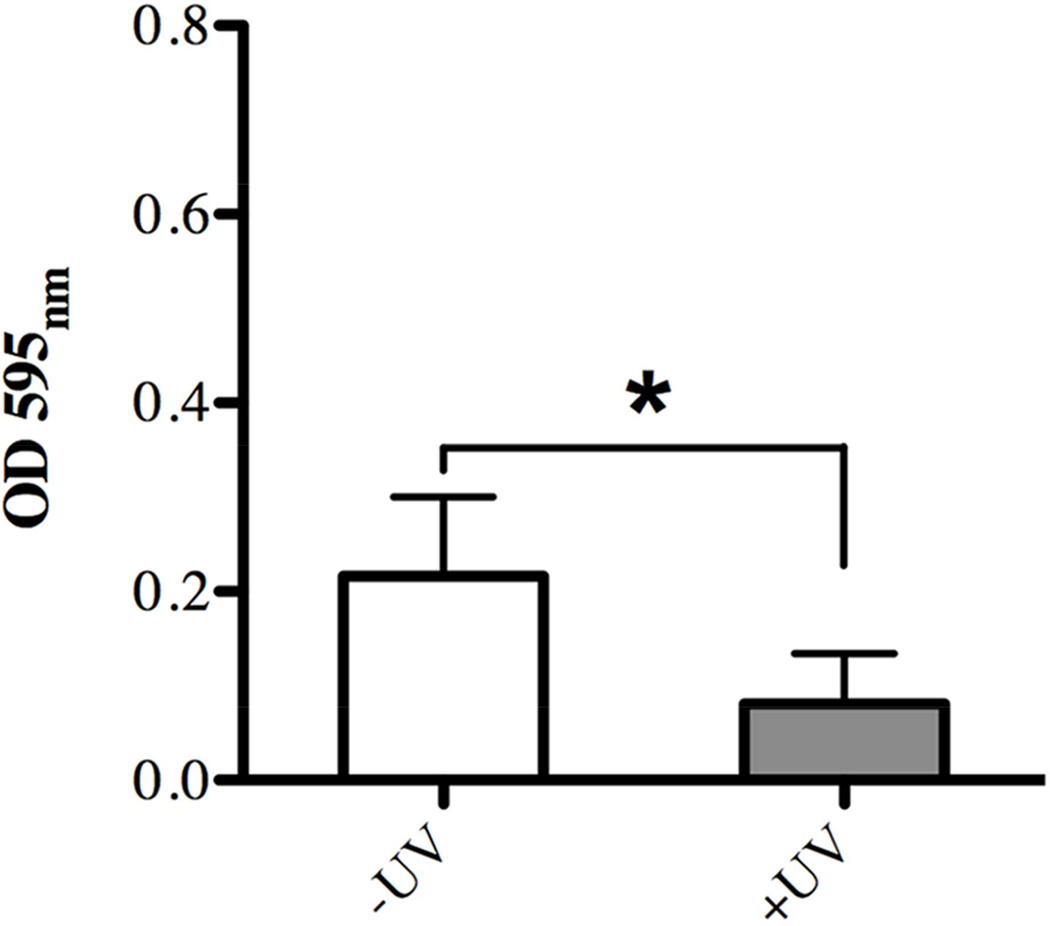
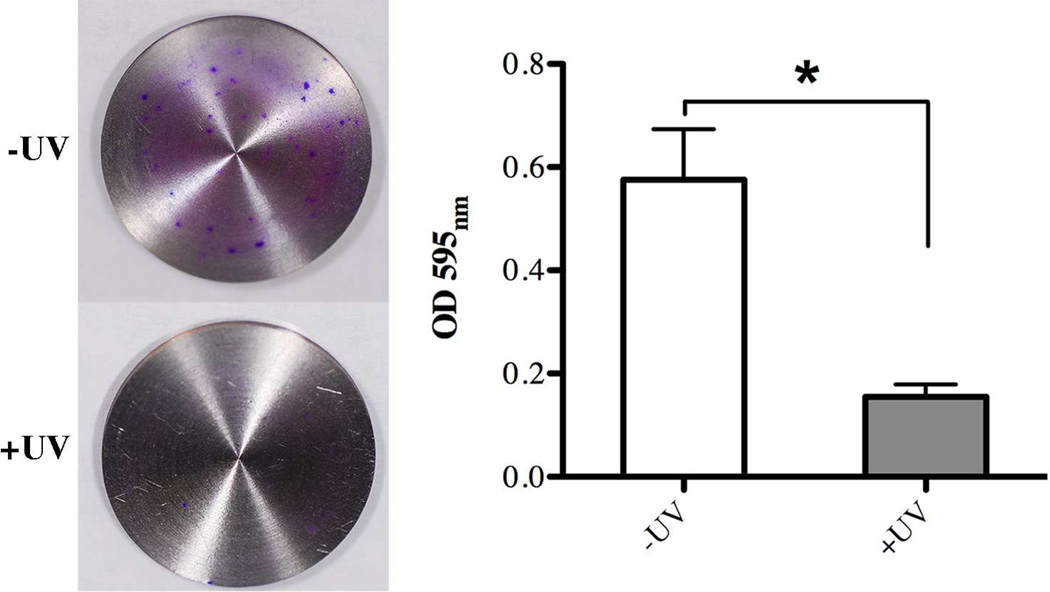
Next, we examined whether the surface property changes of titanium surfaces induced by the 12 min UV-treatment described above had any influence on bacterial attachment and early biofilm formation. The biomasses attached to titanium discs that were subjected to UV-treatment and those without were evaluated after three hours of incubation with a cultured mixture of salivary bacteria using a variety of different approaches. Crystal violet staining disclosed a statistically significant difference in overall bacterial attachment with 2.6-fold less biomass evident on the UV-treated titanium surfaces compared to untreated ones (Fig. 3).
Fig. 3.
Effect of UV-treatment of titanium surfaces on bacterial attachment after 3 hours incubation evaluated via quantitative measurement of crystal violet staining as indicator of biomass accumulation on untreated (white bar, –UV) in comparison to UV-treated (gray bar, +UV) titanium discs. Each value represents the mean ± SD of nine samples comprised of three technical replicates of three independent biological experiments. * indicates a statistically significant difference (p<0.05).
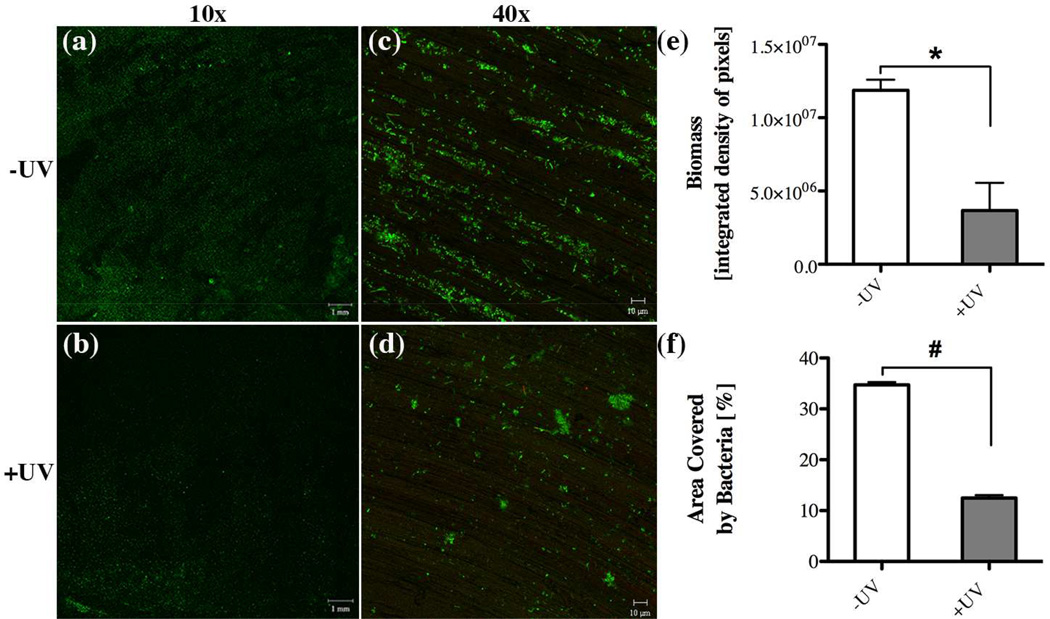
Qualitative assessment of the attached biomass via confocal microscopy confirmed that the UV-treated titanium discs amassed considerably less bacteria compared to their untreated counterparts (Figs. 4a, b). Consistent with the observed difference in bacterial attachment, bacterial colonies on untreated surfaces were generally larger and more abundant (Fig. 4c) compared to the sparse colonization on UV-treated titanium (Fig. 4d). Fluorescent live/dead staining revealed that viability of the attached bacteria was very similar between both types of titanium discs (Fig. 4a–d). Detailed quantitative analysis of the confocal images determined that density and area coverage of the attached biomass was significantly reduced (3.2- and 2.8-fold, respectively) on titanium discs that underwent UV-treatment (Figs. 4e, f), even though the height was not affected (data not shown).
Fig. 4.
Effect of UV-treatment of titanium surfaces on bacterial attachment after 3 hours incubation evaluated via confocal microscopy imaging through (a,b) 10x and (c,d) 40x objectives with representative images illustrating the live/dead distribution of bacterial cells (green for live cells, red for compromised cells) accumulated on (a,c) untreated and (b,d) UV-treated titanium discs. Quantitative comparison of (e) accumulated biomass and (f) the area covered by bacteria between untreated (white bar, –UV) in comparison to UV-treated (gray bar, +UV) titanium discs. Each value represents the mean ± SD of four samples comprised of two technical replicates of two independent biological experiments. Statistically significant differences are indicated as: * p=0.0145, # p=0.0003.
3.3 UV- photofunctionalization treatment reduced bacterial biofilm formation on titanium surfaces
Since our UV-treatment drastically reduced the attachment of salivary bacteria to titanium discs, we evaluated if this effect is sustained during the subsequent biofilm formation and maturation process. Crystal violet staining revealed that after 16 hours of biofilm formation the UV-treated titanium discs had accumulated significantly less (3-fold) biomass compared to untreated discs (Fig. 5).
Fig. 5.
Effect of UV-treatment of titanium surfaces on bacterial biofilm formation after 16 hours incubation evaluated via quantitative measurement of crystal violet staining as indicator of biomass accumulation on untreated (white bar, –UV) in comparison to UV-treated (gray bar, +UV) titanium discs. Each value represents the mean ± SD of nine samples comprised of three technical replicates of three independent biological experiments. * indicates a statistically significant difference (p<0.001).
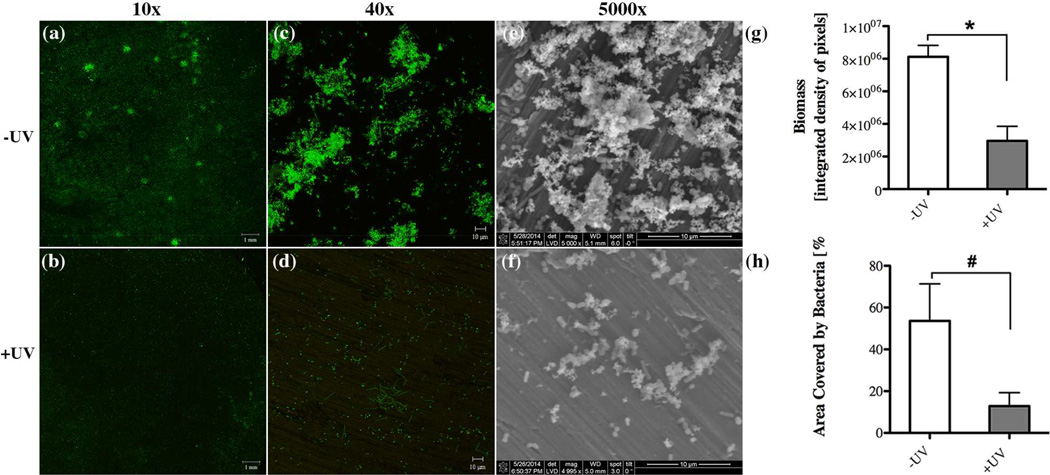
The differences in biofilm formation between UV-treated and untreated titanium discs were confirmed by confocal and scanning electron microscopy analyses. An obvious difference in the overall density of coverage as well as the frequency and size of microcolonies was apparent between the two different surfaces (Figs. 6a, b), while biofilm viability as revealed by a fluorescent live/dead stain was similar. Higher magnification imaging with confocal and scanning electron microscopy revealed that colonization of UV-treated surfaces is clearly more sparse with fewer, smaller and more scattered cell clusters as compared to untreated titanium discs, which were covered with larger, taller and more widespread microcolonies (Figs. 6c–f). Consistent with these qualitative microscopic impressions and above overall biomass determination via crystal violet staining, we found that the average biofilm density and the area coverage of biofilm cells on untreated surfaces were 2.7-fold and 4.2-fold, respectively, higher than on UV-treated titanium discs (Fig. 6g–h).
Fig. 6.
Effect of UV-treatment of titanium surfaces on bacterial biofilm formation after 16 hours incubation evaluated via confocal microscopy imaging through (a,b) 10x and (c,d) 40x objectives with representative images illustrating the live/dead distribution of bacterial cells (green for live cells, red for compromised cells) accumulated on (a,c) untreated (-UV) and (b,d) UV-treated (+UV) titanium discs. Scanning electron microscopy revealing the biofilm structure on (e) -UV and (f) +UV titanium discs. Quantitative comparison of (e) accumulated biomass and (f) the area covered by bacteria between untreated (white bar, –UV) in comparison to UV-treated (gray bar, +UV) titanium discs. Each value represents the mean ± SD of four samples comprised of two technical replicates of two independent biological experiments. Statistically significant differences are indicated as: * p<0.0116), # p<0.0461.
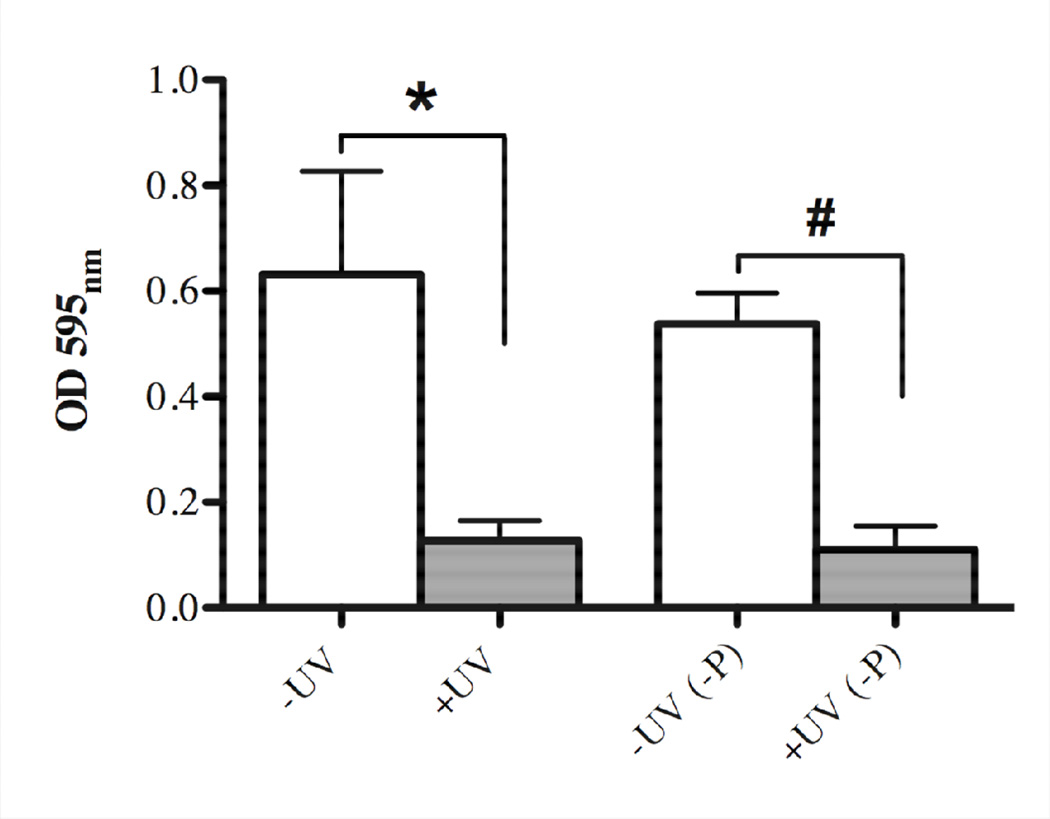
In order to investigate if the effect of UV-photofunctionalization on bacterial biofilm formation is maintained in an aqueous environment, UV-treated and untreated titanium discs were immersed in SHI-FSMS medium for 24 hours prior to biofilm development for 16 hours as described in Material and Methods. Concomitantly, we developed biofilms for 16 hours on UV-treated and untreated titanium surfaces without pre-immersion in medium and evaluated the corresponding biomass accumulation as a control. While there was no meaningful difference between preimmersed and directly used untreated or UV-treated titanium discs, the previously observed significant effect of UV treatment on biofilm reduction was sustained even after pre-incubation of the discs in SHI-FSMS medium for 24 hours (Fig. 7). The differences between the respective untreated and UV-treated titanium surfaces were about 4-fold for both the pre-immersed and the directly used discs (Fig. 7).
Fig. 7.
Sustainability of UV-treatment of titanium surfaces and the effect on 16 hours bacterial biofilm formation after immersion of titanium discs in liquid SHI-FSMS medium for 24 hours prior to incubation with bacteria. Quantitative measurement of crystal violet staining showing biomass accumulation on untreated (white bar, –UV) in comparison to UV-treated (gray bar, +UV) titanium discs for directly used samples and those pre-immersed in liquid SHI-FSMS (-P). Each value represents the mean ± SD of four samples comprised of two technical replicates of two independent biological experiments. Statistically significant differences are indicated as: * p=0.0012), # p<0.0001.
3.5 UV- photofunctionalization treatment does not affect the bacterial biofilm community profile on titanium surfaces
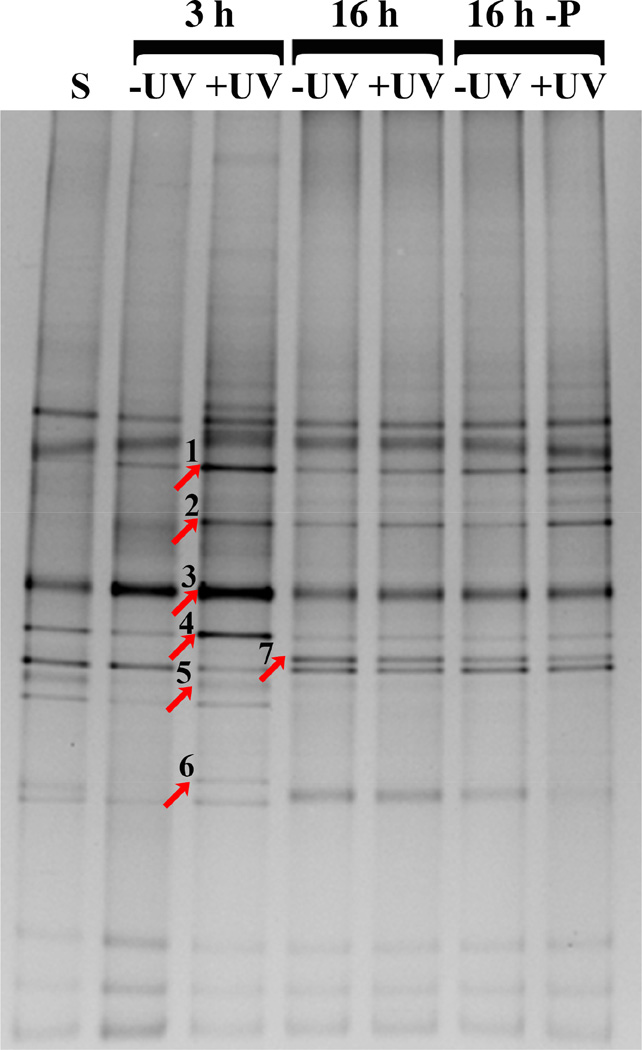
Our next goal was to evaluate whether the UV-treatment in addition to reducing biofilm accumulation had an influence on the type of bacteria that adhered to and formed biofilms on the titanium surfaces. Community profiling via DGGE disclosed that during the 3 hour attachment and early biofilm formation period, the bacterial compositions for untreated and UV-treated surfaces was very similar with a possible difference in the dominance of certain taxa (Fig. 8). The overall profile was maintained in the samples collected after 16 hours of biofilm formation with minor differences in banding pattern compared to the 3 hour samples. Sequencing of selected bands that exhibited different intensities and comparison to the Human oral Microbial Database (HOMD) identified the corresponding microorganisms as Fusobacterium periodonticum (band 1), two Streptococci of the Mitis group (bands 2 and 3), Porphyromonas sp [HOT_279] (band 4), Gemella sp (band 5), Peptostreptococcus stomatis (band 6) and an additional Streptococcus sp (band 7).
Fig. 8.
DGGE analysis of oral microbial communities formed on untreated (-UV) and UV-treated (+UV) titanium discs surfaces after 3 hours and with and without 24 hour pre-immersion in liquid SHI-FSMS (-P) medium after 16 hours. Bands that were excised for DNA sequencing are indicated by an arrow. Microbial identities are as follows: (1) Fusobacterium periodonticum, (2) Streptococcus (Mitis group), (3) Streptococcus (Mitis group), (4) Porphyromonas sp [HOT_279], (5) Gemella sp, (6) Peptostreptococcus stomatis, and (7) Streptococcus sp.
4. Discussion
Photofunctionalization of titanium has previously been demonstrated to provide many benefits to this popular implant material including greatly enhanced osteoblast attachment and proliferation, improved osseointegration as well as reduced attachment of dangerous wound pathogens [17, 26, 30]. The surface property changes induced by UV-treatment such as super-hydrophilicity and removal of hydrocarbon contamination have significant impact on these biological benefits. Typical UV-treatment times, however, last for 48 hours [17, 19, 26, 42], which is prohibitive for convenient chair side application. In this study, we demonstrated that a brief 12 min UV treatment of titanium in a specialized patented photo device is sufficient to alter titanium surface properties (Fig 1) similar to those reported previously for longer treatment times [19, 26]. Even upon this short UV-exposure time, titanium surfaces displayed a drastic change from hydrophobic to very hydrophilic. Importantly, we demonstrated that this surface property change was maintained in an aqueous environment, while it slowly reversed upon exposure to air. Maintenance of surface hydrophilicity after implant placement is a critical factor during the wound healing and bone formation processes to achieve the most favorable outcome by enhancing interaction between the implant material and host cells. The short photofunctionalization treatment employed here was recently shown to result in accelerated and enhanced osseointegration similar to the longer UV-treatment times employed in earlier studies [29, 37, 38, 43].
In addition to maximizing osseointegration, preventing the colonization of implant surfaces with bacteria is another essential consideration in implant surgery. Bacterial contamination during surgery is especially a concern for the placement of dental implants, which occurs in the challenging non-sterile environment of the oral cavity that harbors billions of bacteria comprised of over 600 different species [44, 45]. In contrast to orthopedic implants that are placed sub-merged into sterile tissue, dental implants are placed trans-mucosally in the jaw bone with their upper portion exposed to the oral cavity during healing because they are positioned to support prosthetic teeth. To date polymicrobial infections such as peri-implantitis remain an important cause for implant failure, especially in patients with a previous history of periodontal diseases [5, 46]. To address the potential of our 12 min UV-treatment to improve this critical aspect of implant failure, we investigated if the changes in titanium surface properties had an effect on the attachment and biofilm formation of human oral bacteria. Overall, many pathogenic bacteria have been described as rather being hydrophobic, which plays a critical role in attachment and biofilm formation [47–49]. Ultimately, however, the diversity of oral microbial population and their interspecies interactions as well as the environmental factors modulate the contact with the surface [50, 51]. As a model system, we used a cultivable polymicrobial community representative of the oral cavity [40] in combination with a bacterial growth medium (SHI-FSMS) containing saliva and blood components that are typically present during dental implant surgery. Especially salivary proteins are known to play important roles in the surface attachment of oral bacteria and many species have evolved specialized adhesins that specifically recognize distinct saliva components [52, 53]. We demonstrated via several independent approaches that the convenient 12 min photofunctionalization of machined titanium reduced the amount of oral community-derived bacteria that attach to the surface by about 3 to 4-fold during the critical period after the initial implant placement, when the bone-generating osteoblasts compete with microorganisms for space on the newly available surface. This reduction in biomass accumulation was also reflected by reduced biofilm density and surface area coverage, even though bacterial viability was not affected. These results are very encouraging, considering the importance of surface availability in the competition between bacterial and eukaryotic cell attachment [54]. These findings are consistent with a previous study by Yamada and co-workers, who examined the early stages of biomass accumulation by two important wound pathogens, Staphylococcus aureus and Streptococcus pyogenes, on different titanium surfaces subjected to UVA and UVC irradiation for 48 hours in a monospecies culture model [30].
Notably, this reduction in bacterial attachment and biofilm formation on the UV-treated titanium surface is sustained for at least 16 hours and thus well beyond the six hours post-implantation period during which implants are considered to be particularly susceptible to bacterial colonization [55, 56]. We also observed that the biomass attached to UV-treated titanium remained sparse, predominantly consisting of individual attached cells and small cell clusters compared to untreated surfaces that were mostly covered in maturing biofilms comprised of large colonies that appeared to be encased in matrix material. In addition to preventing host cell attachment to the implant by competing for space, biofilms have also been shown to elicit much stronger inflammatory response – an important reason for bone loss – compared to planktonic bacteria [57, 58]. Furthermore, bacteria contaminating titanium implants have been demonstrated to migrate into and infect surrounding tissues, while apparently not being cleared by macrophages [59]. A weaker immune response against biofilm cells compared to their planktonic counterparts has been described [60, 61] which makes prevention of biofilm formation on implant materials a key factor in averting infections. Another promising result of our study for clinical applications is the finding that even after pre-immersion of the titanium surfaces for 24 hours in the saliva and blood components containing SHI-FSMS medium, the reducing effect of photofunctionalization on biomass accumulation is maintained. This prolonged protective effect against bacterial colonization in combination with the enhancement of osteoblast attachment and proliferation [17, 19, 26, 43] has a strong potential to not only promote continued implant osseointegration but also reduce the risk of implant loss due to bacterial biofilm infections. The time period tested in this study is sufficient to allow for initial wound healing and formation of a protective clot that minimizes bacterial access from the oral cavity to the wound site. Furthermore, if bacteria were able to enter the implant placement site they would not be able to efficiently attach to UV-treated implant surfaces and form a biofilm but rather stay planktonic and thus be easier to clear by the host immune system. Future animal models and clinical studies will be necessary to investigate if this reduction in bacterial attachment and biofilm formation during implant placement in combination with the enhanced osseointegration of UV-treated titanium implants translates into improved long-term clinical outcome of dental implants.
Since photofunctionalization induces a drastic change in titanium surface properties from hydrophobic to superhydrophilic and hydrophobic/philic interaction play an important role in initial bacterial attachment, we examined if the altered surface properties would also affect the profile of bacteria colonizing the untreated versus the UV-treated titanium discs. DGGE analysis revealed a notable difference between UV-treated and untreated surfaces in the bacterial community profile and prevalence of the corresponding microorganisms attached to the respective titanium surfaces during the 3 hours initial attachment period. Surprisingly, streptococci of the Mitis group as well as Porphyromonas sp, which have been described as generally hydrophobic [62] are more prevalent in the communities isolated from the superhydrophilic UV-treated titanium discs. Other studies, however, demonstrated that hydrophobicity of titanium surfaces is not a factor in P. gingivalis and S. sanguinis attachment [63] and that cell surface hydrophobicity of P. gingivalis greatly vary in a strain-dependent manner [64, 65] Fusobacterium periodonticum also appeared to preferentially attach to the UV-treated surfaces, which is consistent with a previous report showing that fusobacteria colonize hydrophilic surfaces at a higher rate [63]. Overall microorganisms have been found to display a wide range of hydrophobicities depending on environmental conditions. Currently, there is not a clear consent on the role of cell surface characteristic in the ability of bacteria to attach to hydrophilic or hydrophobic surfaces, even though hydrophobic interactions generally seem to favor biofilm formation [66]. Furthermore, the salivary and blood components used in our model system that are relevant in the clinical context of implant placement are likely an important factor for the profile of initial bacterial attachment. Especially salivary components are known to provide important binding sites for the surface colonization with oral bacteria [67, 68]. The distinct difference in bacterial profiles between UV-treated and untreated titanium was limited to the initial surface attachment. After 16 hours of biofilm formation, the attached bacterial communities were identical, regardless if the discs were pre-immersed in medium for 24 hours or not. This could be due to the fact that after the initial attachment, bacteria start conditioning the surface with matrix material and thus would mask the actual titanium surface properties. Interestingly, the profiles after 16 hours largely resemble the community attached to untreated titanium surfaces after 3 hours with only the appearance of an additional streptococcus species as a noticeable difference.
In conclusion, we demonstrated in our study that the changes in titanium surface properties induced by a relatively short photofunctionalization treatment leads to a significant reduction in the attachment and biofilm formation by human oral bacteria. Importantly, this effect is maintained in the presence of salivary and blood components, which are typically present in the oral environment of a dental implant placement site during time periods that exceed those considered critical for bacterial contamination and initial wound healing. Therefore, this chairside-friendly application for modification of titanium properties that was already shown to significantly enhance osseointegration in previous studies can provide the additional benefit of reducing bacterial implant contamination during the surgical process. These qualities have a strong potential to significantly reduce dental implant-relaled diseases and failure.
Acknowledgements
This study was supported by a gift from Ushio Inc to Takahiro Ogawa. Renate Lux and Wenyuan Shi were supported by the National Institutes of Health (NIDCR DE021108 and NIDCR DE020102), while Erica Doregatti de Avila was supported by CAPES (Coordination for the Improvement of Higher Level – or Education – Personnel) grant #5603/13-7 and the Lemann Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pjetursson BE, Asgeirsson AG, Zwahlen M, Sailer I. Improvements in implant dentistry over the last decade: comparison of survival and complication rates in older and newer publications. Int J Oral Maxillofac Implants. 2014;29(Suppl):308–324. doi: 10.11607/jomi.2014suppl.g5.2. [DOI] [PubMed] [Google Scholar]
- 2.Simonis P, Dufour T, Tenenbaum H. Long-term implant survival and success: a 10–16-year follow-up of non-submerged dental implants. Clin Oral Implants Res. 2010;21:772–777. doi: 10.1111/j.1600-0501.2010.01912.x. [DOI] [PubMed] [Google Scholar]
- 3.Clementini M, Rossetti PH, Penarrocha D, Micarelli C, Bonachela WC, Canullo L. Systemic risk factors for peri-implant bone loss: a systematic review and meta-analysis. Int J Oral Maxillofac Surg. 2014;43:323–334. doi: 10.1016/j.ijom.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 4.Heitz-Mayfield LJ. Peri-implant diseases: diagnosis and risk indicators. J Clin Periodontol. 2008;35:292–304. doi: 10.1111/j.1600-051X.2008.01275.x. [DOI] [PubMed] [Google Scholar]
- 5.Aguirre-Zorzano LA, Estefania-Fresco R, Telletxea O, Bravo M. Prevalence of peri-implant inflammatory disease in patients with a history of periodontal disease who receive supportive periodontal therapy. Clin Oral Implants Res. 2014 doi: 10.1111/clr.12462. [DOI] [PubMed] [Google Scholar]
- 6.Chrcanovic BR, Albrektsson T, Wennerberg A. Periodontally compromised vs. periodontally healthy patients and dental implants: a systematic review and meta-analysis. J Dent. 2014;42:1509–1527. doi: 10.1016/j.jdent.2014.09.013. [DOI] [PubMed] [Google Scholar]
- 7.Ong CT, Ivanovski S, Needleman IG, Retzepi M, Moles DR, Tonetti MS, et al. Systematic review of implant outcomes in treated periodontitis subjects. J Clin Periodontol. 2008;35:438–462. doi: 10.1111/j.1600-051X.2008.01207.x. [DOI] [PubMed] [Google Scholar]
- 8.Lang NP, Berglundh T Working Group 4 of Seventh European Workshop on P. Periimplant diseases: where are we now?--Consensus of the Seventh European Workshop on Periodontology. J Clin Periodontol. 2011;38(Suppl 11):178–181. doi: 10.1111/j.1600-051X.2010.01674.x. [DOI] [PubMed] [Google Scholar]
- 9.Busscher HJ, Rinastiti M, Siswomihardjo W, van der Mei HC. Biofilm formation on dental restorative and implant materials. J Dent Res. 2010;89:657–665. doi: 10.1177/0022034510368644. [DOI] [PubMed] [Google Scholar]
- 10.Lee A, Wang HL. Biofilm related to dental implants. Implant Dent. 2010;19:387–393. doi: 10.1097/ID.0b013e3181effa53. [DOI] [PubMed] [Google Scholar]
- 11.Park JW, Jang JH, Lee CS, Hanawa T. Osteoconductivity of hydrophilic microstructured titanium implants with phosphate ion chemistry. Acta Biomater. 2009;5:2311–2321. doi: 10.1016/j.actbio.2009.02.026. [DOI] [PubMed] [Google Scholar]
- 12.Mullen MD, Wolf DC, Ferris FG, Beveridge TJ, Flemming CA, Bailey GW. Bacterial sorption of heavy metals. Appl Environ Microbiol. 1989;55:3143–3149. doi: 10.1128/aem.55.12.3143-3149.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jin L, Guo W, Xue P, Gao H, Zhao M, Zheng C, et al. Quantitative assay for the colonization ability of heterogeneous bacteria on controlled nanopillar structures. Nanotechnology. 2015;26:055702. doi: 10.1088/0957-4484/26/5/055702. [DOI] [PubMed] [Google Scholar]
- 14.Cavalcanti IM, Ricomini Filho AP, Lucena-Ferreira SC, da Silva WJ, Paes Leme AF, Senna PM, et al. Salivary pellicle composition and multispecies biofilm developed on titanium nitrided by cold plasma. Arch Oral Biol. 2014;59:695–703. doi: 10.1016/j.archoralbio.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 15.Sela MN, Badihi L, Rosen G, Steinberg D, Kohavi D. Adsorption of human plasma proteins to modified titanium surfaces. Clin Oral Implants Res. 2007;18:630–638. doi: 10.1111/j.1600-0501.2007.01373.x. [DOI] [PubMed] [Google Scholar]
- 16.Badihi Hauslich L, Sela MN, Steinberg D, Rosen G, Kohavi D. The adhesion of oral bacteria to modified titanium surfaces: role of plasma proteins and electrostatic forces. Clin Oral Implants Res. 2013;24(Suppl A100):49–56. doi: 10.1111/j.1600-0501.2011.02364.x. [DOI] [PubMed] [Google Scholar]
- 17.Tsukimura N, Yamada M, Iwasa F, Minamikawa H, Att W, Ueno T, et al. Synergistic effects of UV photofunctionalization and micro-nano hybrid topography on the biological properties of titanium. Biomaterials. 2011;32:4358–4368. doi: 10.1016/j.biomaterials.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 18.Iwasa F, Tsukimura N, Sugita Y, Kanuru RK, Kubo K, Hasnain H, et al. TiO2 micro-nano-hybrid surface to alleviate biological aging of UV-photofunctionalized titanium. Int J Nanomedicine. 2011;6:1327–1341. doi: 10.2147/IJN.S22099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aita H, Hori N, Takeuchi M, Suzuki T, Yamada M, Anpo M, et al. The effect of ultraviolet functionalization of titanium on integration with bone. Biomaterials. 2009;30:1015–1025. doi: 10.1016/j.biomaterials.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 20.Andersson B, Odman P, Lindvall AM, Lithner B. Single-tooth restorations supported by osseointegrated implants: results and experiences from a prospective study after 2 to 3 years. Int J Oral Maxillofac Implants. 1995;10:702–711. [PubMed] [Google Scholar]
- 21.Branemark PI. Osseointegration and its experimental background. J Prosthet Dent. 1983;50:399–410. doi: 10.1016/s0022-3913(83)80101-2. [DOI] [PubMed] [Google Scholar]
- 22.Horasawa N, Yamashita T, Uehara S, Udagawa N. High-performance scaffolds on titanium surfaces: osteoblast differentiation and mineralization promoted by a globular fibrinogen layer through cell-autonomous BMP signaling. Mater Sci Eng C Mater Biol Appl. 2015;46:86–96. doi: 10.1016/j.msec.2014.10.025. [DOI] [PubMed] [Google Scholar]
- 23.Salou L, Hoornaert A, Louarn G, Layrolle P. Enhanced osseointegration of titanium implants with nanostructured surfaces: an experimental study in rabbits. Acta Biomater. 2015;11:494–502. doi: 10.1016/j.actbio.2014.10.017. [DOI] [PubMed] [Google Scholar]
- 24.Funato A, Ogawa T. Photofunctionalized dental implants: a case series in compromised bone. Int J Oral Maxillofac Implants. 2013;28:1589–1601. doi: 10.11607/jomi.3232. [DOI] [PubMed] [Google Scholar]
- 25.Funato A, Yamada M, Ogawa T. Success rate, healing time, and implant stability of photofunctionalized dental implants. Int J Oral Maxillofac Implants. 2013;28:1261–1271. doi: 10.11607/jomi.3263. [DOI] [PubMed] [Google Scholar]
- 26.Miyauchi T, Yamada M, Yamamoto A, Iwasa F, Suzawa T, Kamijo R, et al. The enhanced characteristics of osteoblast adhesion to photofunctionalized nanoscale TiO2 layers on biomaterials surfaces. Biomaterials. 2010;31:3827–3839. doi: 10.1016/j.biomaterials.2010.01.133. [DOI] [PubMed] [Google Scholar]
- 27.Minamikawa H, Ikeda T, Att W, Hagiwara Y, Hirota M, Tabuchi M, et al. Photofunctionalization increases the bioactivity and osteoconductivity of the titanium alloy Ti6Al4V. J Biomed Mater Res A. 2014;102:3618–3630. doi: 10.1002/jbm.a.35030. [DOI] [PubMed] [Google Scholar]
- 28.Pyo SW, Park YB, Moon HS, Lee JH, Ogawa T. Photofunctionalization enhances bone-implant contact, dynamics of interfacial osteogenesis, marginal bone seal, and removal torque value of implants: a dog jawbone study. Implant Dent. 2013;22:666–675. doi: 10.1097/ID.0000000000000003. [DOI] [PubMed] [Google Scholar]
- 29.Sugita Y, Honda Y, Kato I, Kubo K, Maeda H, Ogawa T. Role of photofunctionalization in mitigating impaired osseointegration associated with type 2 diabetes in rats. Int J Oral Maxillofac Implants. 2014;29:1293–1300. doi: 10.11607/jomi.3480. [DOI] [PubMed] [Google Scholar]
- 30.Yamada Y, Yamada M, Ueda T, Sakurai K. Reduction of biofilm formation on titanium surface with ultraviolet-C pre-irradiation. J Biomater Appl. 2013 doi: 10.1177/0885328213518085. [DOI] [PubMed] [Google Scholar]
- 31.Siqueira JF, Jr, Fouad AF, Rocas IN. Pyrosequencing as a tool for better understanding of human microbiomes. J Oral Microbiol. 2012:4. doi: 10.3402/jom.v4i0.10743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Safii SH, Palmer RM, Wilson RF. Risk of implant failure and marginal bone loss in subjects with a history of periodontitis: a systematic review and meta-analysis. Clin Implant Dent Relat Res. 2010;12:165–174. doi: 10.1111/j.1708-8208.2009.00162.x. [DOI] [PubMed] [Google Scholar]
- 33.Mombelli A, Muller N, Cionca N. The epidemiology of peri-implantitis. Clin Oral Implants Res. 2012;23(Suppl 6):67–76. doi: 10.1111/j.1600-0501.2012.02541.x. [DOI] [PubMed] [Google Scholar]
- 34.Kohavi D, Klinger A, Steinberg D, Mann E, Sela NM. alpha-Amylase and salivary albumin adsorption onto titanium, enamel and dentin: an in vivo study. Biomaterials. 1997;18:903–906. doi: 10.1016/s0142-9612(97)00026-4. [DOI] [PubMed] [Google Scholar]
- 35.Att W, Hori N, Takeuchi M, Ouyang J, Yang Y, Anpo M, et al. Time-dependent degradation of titanium osteoconductivity: an implication of biological aging of implant materials. Biomaterials. 2009;30:5352–5363. doi: 10.1016/j.biomaterials.2009.06.040. [DOI] [PubMed] [Google Scholar]
- 36.Hori N, Att W, Ueno T, Sato N, Yamada M, Saruwatari L, et al. Age-dependent degradation of the protein adsorption capacity of titanium. J Dent Res. 2009;88:663–667. doi: 10.1177/0022034509339567. [DOI] [PubMed] [Google Scholar]
- 37.Ishijima M, Hirota M, Park W, Honda MJ, Tsukimura N, Isokawa K, et al. Osteogenic cell sheets reinforced with photofunctionalized micro-thin titanium. J Biomater Appl. 2015 doi: 10.1177/0885328214567693. [DOI] [PubMed] [Google Scholar]
- 38.Hirota M, Ikeda T, Tabuchi M, Iwai T, Tohnai I, Ogawa T. Effect of ultraviolet-mediated photofunctionalization for bone formation around medical titanium mesh. J Oral Maxillofac Surg. 2014;72:1691–1702. doi: 10.1016/j.joms.2014.05.012. [DOI] [PubMed] [Google Scholar]
- 39.Edlund A, Yang Y, Hall AP, Guo L, Lux R, He X, et al. An in vitro biofilm model system maintaining a highly reproducible species and metabolic diversity approaching that of the human oral microbiome. Microbiome. 2013;1:25. doi: 10.1186/2049-2618-1-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tian Y, He X, Torralba M, Yooseph S, Nelson KE, Lux R, et al. Using DGGE profiling to develop a novel culture medium suitable for oral microbial communities. Mol Oral Microbiol. 2010;25:357–367. doi: 10.1111/j.2041-1014.2010.00585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rupf S, Merte K, Eschrich K. Quantification of bacteria in oral samples by competitive polymerase chain reaction. J Dent Res. 1999;78:850–856. doi: 10.1177/00220345990780040501. [DOI] [PubMed] [Google Scholar]
- 42.Hori N, Iwasa F, Tsukimura N, Sugita Y, Ueno T, Kojima N, et al. Effects of UV photofunctionalization on the nanotopography enhanced initial bioactivity of titanium. Acta Biomater. 2011;7:3679–3691. doi: 10.1016/j.actbio.2011.06.022. [DOI] [PubMed] [Google Scholar]
- 43.Ueno T, Yamada M, Hori N, Suzuki T, Ogawa T. Effect of ultraviolet photoactivation of titanium on osseointegration in a rat model. Int J Oral Maxillofac Implants. 2010;25:287–294. [PubMed] [Google Scholar]
- 44.Paster BJ, Olsen I, Aas JA, Dewhirst FE. The breadth of bacterial diversity in the human periodontal pocket and other oral sites. Periodontol 2000. 2006;42:80–87. doi: 10.1111/j.1600-0757.2006.00174.x. [DOI] [PubMed] [Google Scholar]
- 45.Paster BJ, Boches SK, Galvin JL, Ericson RE, Lau CN, Levanos VA, et al. Bacterial diversity in human subgingival plaque. J Bacteriol. 2001;183:3770–3783. doi: 10.1128/JB.183.12.3770-3783.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saaby M, Karring E, Schou S, Isidor F. Factors influencing severity of peri-implantitis. Clin Oral Implants Res. 2014 doi: 10.1111/clr.12505. [DOI] [PubMed] [Google Scholar]
- 47.Cerca N, Pier GB, Vilanova M, Oliveira R, Azeredo J. Quantitative analysis of adhesion and biofilm formation on hydrophilic and hydrophobic surfaces of clinical isolates of Staphylococcus epidermidis. Res Microbiol. 2005;156:506–514. doi: 10.1016/j.resmic.2005.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Makin SA, Beveridge TJ. The influence of A-band and B-band lipopolysaccharide on the surface characteristics and adhesion of Pseudomonas aeruginosa to surfaces. Microbiology. 1996;142(Pt 2):299–307. doi: 10.1099/13500872-142-2-299. [DOI] [PubMed] [Google Scholar]
- 49.Langley S, Beveridge TJ. Effect of O-side-chain-lipopolysaccharide chemistry on metal binding. Appl Environ Microbiol. 1999;65:489–498. doi: 10.1128/aem.65.2.489-498.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Caiazza NC, O'Toole GA. SadB is required for the transition from reversible to irreversible attachment during biofilm formation by Pseudomonas aeruginosa PA14. J Bacteriol. 2004;186:4476–4485. doi: 10.1128/JB.186.14.4476-4485.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ng WL, Bassler BL. Bacterial quorum-sensing network architectures. Annu Rev Genet. 2009;43:197–222. doi: 10.1146/annurev-genet-102108-134304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hong SW, Seo DG, Baik JE, Cho K, Yun CH, Han SH. Differential profiles of salivary proteins with affinity to Streptococcus mutans lipoteichoic acid in caries-free and caries-positive human subjects. Mol Oral Microbiol. 2014;29:208–218. doi: 10.1111/omi.12057. [DOI] [PubMed] [Google Scholar]
- 53.Schenkels LC, Walgreen-Weterings E, Oomen LC, Bolscher JG, Veerman EC, Nieuw Amerongen AV. In vivo binding of the salivary glycoprotein EP-GP (identical to GCDFP-15) to oral and non-oral bacteria detection and identification of EP-GP binding species. Biol Chem. 1997;378:83–88. doi: 10.1515/bchm.1997.378.2.83. [DOI] [PubMed] [Google Scholar]
- 54.Gristina AG. Biomaterial-centered infection: microbial adhesion versus tissue integration. Science. 1987;237:1588–1595. doi: 10.1126/science.3629258. [DOI] [PubMed] [Google Scholar]
- 55.Hetrick EM, Schoenfisch MH. Reducing implant-related infections: active release strategies. Chem Soc Rev. 2006;35:780–789. doi: 10.1039/b515219b. [DOI] [PubMed] [Google Scholar]
- 56.Poelstra KA, Barekzi NA, Rediske AM, Felts AG, Slunt JB, Grainger DW. Prophylactic treatment of gram-positive and gram-negative abdominal implant infections using locally delivered polyclonal antibodies. J Biomed Mater Res. 2002;60:206–215. doi: 10.1002/jbm.10069. [DOI] [PubMed] [Google Scholar]
- 57.Secor PR, James GA, Fleckman P, Olerud JE, McInnerney K, Stewart PS. Staphylococcus aureus Biofilm and Planktonic cultures differentially impact gene expression, mapk phosphorylation, and cytokine production in human keratinocytes. BMC Microbiol. 2011;11:143. doi: 10.1186/1471-2180-11-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tankersley A, Frank MB, Bebak M, Brennan R. Early effects of Staphylococcus aureus biofilm secreted products on inflammatory responses of human epithelial keratinocytes. J Inflamm (Lond) 2014;11:17. doi: 10.1186/1476-9255-11-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Riool M, de Boer L, Jaspers V, van der Loos CM, van Wamel WJ, Wu G, et al. Staphylococcus epidermidis originating from titanium implants infects surrounding tissue and immune cells. Acta Biomater. 2014;10:5202–5212. doi: 10.1016/j.actbio.2014.08.012. [DOI] [PubMed] [Google Scholar]
- 60.Leid JG, Kerr M, Selgado C, Johnson C, Moreno G, Smith A, et al. Flagellum-mediated biofilm defense mechanisms of Pseudomonas aeruginosa against host-derived lactoferrin. Infect Immun. 2009;77:4559–4566. doi: 10.1128/IAI.00075-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Thurlow LR, Hanke ML, Fritz T, Angle A, Aldrich A, Williams SH, et al. Staphylococcus aureus biofilms prevent macrophage phagocytosis and attenuate inflammation in vivo. J Immunol. 2011;186:6585–6596. doi: 10.4049/jimmunol.1002794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gibbons RJ, Etherden I, Moreno EC. Association of neuraminidase-sensitive receptors and putative hydrophobic interactions with high-affinity binding sites for Streptococcus sanguis C5 in salivary pellicles. Infect Immun. 1983;42:1006–1012. doi: 10.1128/iai.42.3.1006-1012.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Almaguer-Flores A, Olivares-Navarrete R, Wieland M, Ximenez-Fyvie LA, Schwartz Z, Boyan BD. Influence of topography and hydrophilicity on initial oral biofilm formation on microstructured titanium surfaces in vitro. Clin Oral Implants Res. 2012;23:301–307. doi: 10.1111/j.1600-0501.2011.02184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Naito Y, Ohno J, Takazoe I, Okuda K. The relationship between polysaccharide antigen and interleukin-1 beta producing activity in Porphyromonas gingivalis. Bull Tokyo Dent Coll. 1992;33:187–195. [PubMed] [Google Scholar]
- 65.Naito Y, Tohda H, Okuda K, Takazoe I. Adherence and hydrophobicity of invasive and noninvasive strains of Porphyromonas gingivalis. Oral Microbiol Immunol. 1993;8:195–202. doi: 10.1111/j.1399-302x.1993.tb00559.x. [DOI] [PubMed] [Google Scholar]
- 66.Krasowska A, Sigler K. How microorganisms use hydrophobicity and what does this mean for human needs? Front Cell Infect Microbiol. 2014;4:112. doi: 10.3389/fcimb.2014.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lenander-Lumikari M, Loimaranta V. Saliva and dental caries. Adv Dent Res. 2000;14:40–47. doi: 10.1177/08959374000140010601. [DOI] [PubMed] [Google Scholar]
- 68.Koscielniak D, Jurczak A, Zygmunt A, Krzysciak W. Salivary proteins in health and disease. Acta Biochim Pol. 2012;59:451–457. [PubMed] [Google Scholar]