Figure 4.
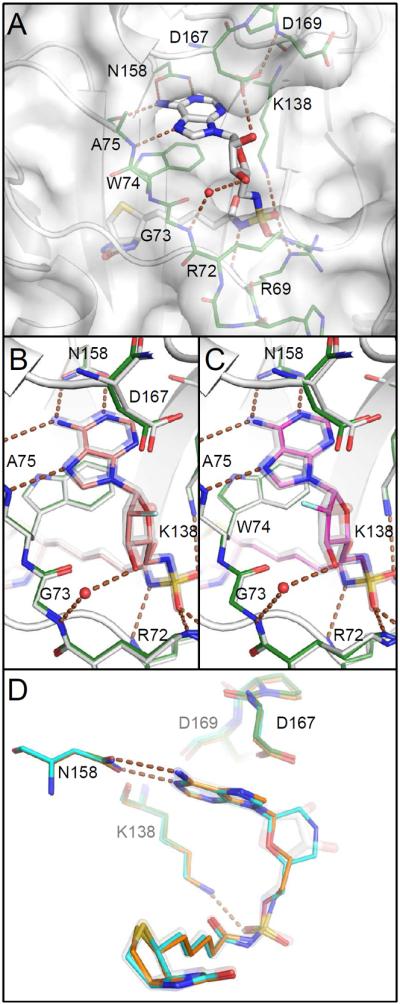
Comparison of co-crystal structures. A) Bio-AMS (14, white) binds with adenosyl and biotinyl groups in deep pockets with Trp74 stacked between them. Protein carbon atoms of this complex are green included in other panels for comparison. Hydrogen bonds are illustrated with brown dashed lines. The ribose is positioned across the open mouth of a larger unoccupied space. B and C) Binding site and ribose ring pucker are unchanged upon binding of the 2′ fluoro analogs with standard (63, salmon) or reversed (69, magenta) stereochemistry, despite the loss of the hydrogen bond to Asp167. Azido analogs (46, 57) bind similarly (not shown). D) Morpholino (82, cyan) and acyclo (90, orange) analogs adopt conformations that mimic the ribose.
