FIGURE 1. Western Blot and Pro-Q analysis to assess the phosphorylation status of myofilament filament proteins in WT and KO heart preparations.
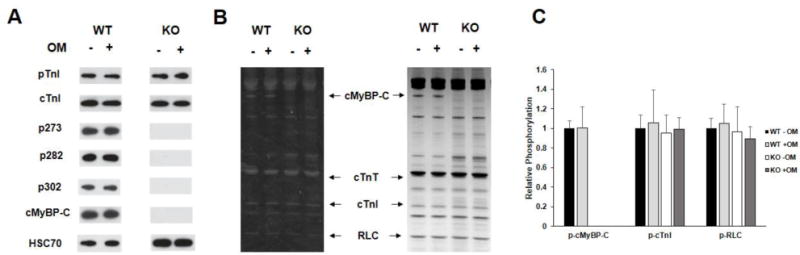
(A) Western blots showing cMyBP-C and TnI phosphorylation before and after treatment with OM in WT and KO heart samples. TnI phosphorylation at residues Ser23/24 was similar between WT and KO samples and was unaffected by OM treatment. cMyBP-C phosphorylation of residues Ser273, Ser282, and Ser302 was absent in KO tissue, and their phosphorylation levels in WT was unaffected by OM treatment. (B) Representative Pro-Q Diamond-stained (left) and Coomassie stained (right) SDS gel showing the phosphorylation status of myofilament proteins before and after treatment with OM in WT and KO heart samples. (C) Quantification of protein phosphorylation as determined by Pro-Q and Coomassie stains from 6 WT and 6 KO hearts. The intensity of the phosphorylation signaling was normalized to the intensity of the total protein signal and the untreated WT myofibril protein phosphorylation was set to 1 as done in our previous study [35]. cMyBP-C phosphorylation was unaffected by OM treatment in WT preparations and was absent in KO preparations. No differences in phosphorylation status of myofilament proteins were observed between WT and KO hearts. Furthermore, treatment with OM for 2 minutes did not induce any significant changes in the phosphorylation status of myofilament proteins in WT and KO hearts. WT, wild-type; KO, knockout; cMyBP-C, cardiac myosin binding protein-C; cTnT, cardiac troponin T; cTnI, cardiac troponin I; RLC, regulatory light chain.
