Abstract
A large percentage of eukaryotic genomes consist of repetitive DNA that plays an important role in the organization, size and evolution. In the case of crickets, chromosomal variability has been found using classical cytogenetics, but almost no information concerning the organization of their repetitive DNAs is available. To better understand the chromosomal organization and diversification of repetitive DNAs in crickets, we studied the chromosomes of two Gryllidae species with highly divergent karyotypes, i.e., 2n(♂) = 29,X0 (Gryllus assimilis) and 2n = 9, neo-X1X2Y (Eneoptera surinamensis). The analyses were performed using classical cytogenetic techniques, repetitive DNA mapping and genome-size estimation. Conserved characteristics were observed, such as the occurrence of a small number of clusters of rDNAs and U snDNAs, in contrast to the multiple clusters/dispersal of the H3 histone genes. The positions of U2 snDNA and 18S rDNA are also conserved, being intermingled within the largest autosome. The distribution and base-pair composition of the heterochromatin and repetitive DNA pools of these organisms differed, suggesting reorganization. Although the microsatellite arrays had a similar distribution pattern, being dispersed along entire chromosomes, as has been observed in some grasshopper species, a band-like pattern was also observed in the E. surinamensis chromosomes, putatively due to their amplification and clustering. In addition to these differences, the genome of E. surinamensis is approximately 2.5 times larger than that of G. assimilis, which we hypothesize is due to the amplification of repetitive DNAs. Finally, we discuss the possible involvement of repetitive DNAs in the differentiation of the neo-sex chromosomes of E. surinamensis, as has been reported in other eukaryotic groups. This study provided an opportunity to explore the evolutionary dynamics of repetitive DNAs in two non-model species and will contribute to the understanding of chromosomal evolution in a group about which little chromosomal and genomic information is known.
Introduction
The Orthoptera order comprises more than 25,700 species, which form the most diverse group of polyneopteran insects [1,2,3]. Variability in the dipoid number has been documented in distinct lineages, which range in the most highly studied groups from 2n(♂) = 15 to 2n(♂) = 35 in katydids Tettigoniidae [4,5,6], from 2n(♂) = 8 to 2n(♂) = 23 in Acrididae grasshoppers [7,8,9] and from 2n(♂) = 7 to 2n(♂) = 29 in Gryllidae crickets [9,10,11,12,13], although the modal number of chromosomes has been documented, such as 2n(♂) = 23 in grasshoppers and 2n = 21 in Gryllidae crickets [8,9]. This extreme karyotypic divergence has been attributed to multiple major chromosomal restructuring events, such as centric fusions, tandem fusions, reciprocal translocations, dissociations and inversions involving autosomes and sex chromosomes [8,9]. In addition to these rearrangements being responsible for the variations in the diploid number, they also caused diversification of the sex chromosomes, originating distinct sex systems, such as neo-XY, neo-X1X2Y and X1X20, with the origin attributed from the X0, which is considered atavic and modal for the group [8,9,14,15,16,17].
Large portions of eukaryotic genomes are composed of sequences that are repeated hundred to thousand times, which are called repetitive DNAs. These sequences could be tandemly arrayed as microsatellites, minisatellites and satellite DNAs (satDNAs) or occur in a scattered pattern, as transposons and retrotransposons [18,19,20,21,22]. In accordance with the selfish DNA hypothesis [23,24], some of these sequences have been maintained in the genome due to their ability to colonize genomic regions lacking or with a low level of recombination activity. Thus, these repeats tend to be highly abundant and ubiquitous in certain chromosomal compartments, such as telomeric and centromeric heterochromatin and non-recombining regions of the sex chromosomes. Repetitive DNAs generally are subjected to replication and expansion processes through multiple mechanisms, such as unequal cross-over, gene conversion, rolling-circle replication, whole-genome duplication, segmental duplications and transposition [18,21,25,26,27,28,29,30]. Because the size and abundance of the repeats vary greatly within and between species, repetitive DNAs are the major cause of variation in the size of eukaryote genomes, and they have also been involved in genomic evolution [18,31,32,33].
Orthopteran species frequently have large genomes, ranging in size from 1.52 Gb in the cave cricket Hadenoecus subterraneus [34] to 16.56 Gb in the grasshopper Podisma pedestris [35]. In some Orthopteran species, the genomes are rich in repetitive DNAs, as for example, in Locusta migratoria (genome size 6.3 Gb [36]) and Schistocerca gregaria grasshoppers (genome size 8.55 Gb [37]). Although the sizes of the genomes of cricket species are generally smaller than those of grasshoppers, they have large genomes compared with those of other insects (see [38,39]); however, very little information concerning the chromosomal organization of the repetitive DNAs in this group is available (see for example [13,17]).
Considering the large genomes rich in repetitive DNAs of some Orthopteran species and the possible role of these sequences in chromosomal evolution, in this study, we aimed to contribute to the understanding of the organization of repetitive DNAs in autosomes and their contribution to the structure of the derived sex chromosomes in crickets. For this purpose, we used two species belonging to the family Gryllidae that have highly divergent karyotypes, i.e., Gryllus assimilis, with 2n(♂) = 29,X0 [40], and Eneoptera surinamensis, with 2n(♂) = 9,neo-X1X2Y [12]. These genomes were compared using flow cytometric genome-size estimation, classical cytogenetic analysis and fluorescent in situ hybridization (FISH) using various repetitive DNAs as probes, such as multigene families (18S and 5S rDNA, U1 and U2 snDNAs and H3 histone), highly and moderately repetitive DNA fractions (C 0 t-DNA), the classical insect telomeric repeat (TTAGG) and 16 microsatellite motifs.
Materials and Methods
Samples, classical chromosomal analysis and banding
Five male and seven female Eneoptera surinamensis were collected in the Parque Estadual Edmundo Navarro de Andrade (Rio Claro, SP, Brazil) between May 2013 and March 2014 with the authorization of COTEC (process number 341/2013) and were maintained in captivity until ovipositioning occurred, whereas Gryllus assimilis animals were obtained from a pool of individuals that had been bred in the biotery of the Univ. Estadual Paulista—UNESP (Rio Claro, SP, Brazil). The embryo preparations for chromosome obtaining were prepared according to Webb et al. [41], with slight modifications of the fixation materials, as follows: After hypotonization was accomplished, the same volume of Carnoy’s modified solution (3:1, absolute ethanol:acetic acid) was applied for 15 minutes, then the embryos were transferred to fresh Carnoy’s solution and were stored in a -20°C freezer until use. At least 60 embryos of each species were used for cytological preparations. In addition, four adult male testes of each species were dissected and were fixed in Carnoy’s modified solution. Adult specimens (ten of each species) were stored in 100% ethanol for subsequent DNA extraction.
Karyological studies were performed using conventional staining with 5% Giemsa solution to confirm the previous karyotypic descriptions. The C-banding procedure was conducted according to Sumner [42], and fluorochrome staining (CMA3/DA/DAPI) to identify G+C or A+T rich regions was performed as described by Schweizer et al. [43]. Female and male genomic DNA was extracted from femurs using the phenol/chloroform-based procedure described in Sambrook and Russel [44].
Probes for repetitive DNAs
The DNA probes for the 5S rRNA and H3 genes were obtained through polymerase chain reaction (PCR) amplification using genomic DNA from Abracris flavolineata as a template and the primers described by Cabral-de-Mello et al. [45] and Colgan et al. [46] for the 5S rRNA gene and the H3 gene, respectively. The sequences of the U snDNAs of Rhammatocerus brasiliensis were obtained using the primers described by Cabral-de-Mello et al. [47] for U1 snDNA and those of Bueno et al. [48] for U2 snDNA. These sequences were previously used as probes and are deposited in GenBank under accession numbers KC936996 (5S rDNA), KC896792 (H3 histone gene), KC896793 (U1 snDNA) and KC896794 (U2 snDNA). The 18S rDNA probe was obtained from a cloned fragment previously isolated from the genome of the Dichotomius semisquamosus beetle (GenBank accession number GQ443313 [45]).
The repetitive DNA-enriched samples were obtained based on the renaturation kinetics of C 0 t-DNA (DNA enriched for highly and moderately repetitive DNA sequences), according to the protocol of Zwick et al. [49], with modifications [45]. It was denaturated 150 ng/μL of fragmented genomic DNA at 95°C. The reassociation time of 25 min was used for both species. The DNA was purified/extracted using a traditional phenol-chloroform procedure [44].
The telomeric probe was obtained through PCR using the self-complementary primers (TTAGG)5 and (CCTAA)5 according to Ijdo et al. [50]. Finally, the probes for the microsatellite motifs were directly labeled at the 5’ end with biotin-14 dATP (Sigma-Aldrich, St Louis, MO, USA) during their synthesis, including probes for mononucleotides (A)30 and (C)30, dinucleotides, (CA)15, (CG)15, (TA)15 and (AG)10, trinucleotides (CAA)10, (CAC)10, (TAA)10, (GAA)10, (CGG)10, (CAG)10, (TAC)10 and (GAG)10, tetranucleotides (GACA)4 and (GATA)8.
Fluorescence in situ hybridization (FISH)
The non-cloned 5S rDNA and U snDNAs sequences and telomeric probes were labeled through PCR using digoxigenin-11-dUTP (Roche, Mannheim, Germany). Plasmids containing the 18S rRNA gene or H3 histone gene and the C 0 t-DNA fraction were labeled via nick translation using biotin-14-dATP (Invitrogen, San Diego, CA, USA). Single or two-color FISH of mitotic cells was performed according to Pinkel et al. [51], with modifications [45]. Fiber-FISH was conducted as described in de Barros et al. [52] and Camacho et al. [53] using suspensions of testis cells. Probes labeled with digoxigenin-11-dUTP were detected using rhodamine-conjugated anti-digoxigenin (Roche), and probes labeled with biotin-14-dATP were detected using Streptavidin Alexa Fluor 488-conjugated (Invitrogen).
The preparations were counterstained using 4’,6-diamidine-2’-phenylindole dihydrochloride (DAPI) and were mounted in Vectashield (Vector, Burlingame, CA, USA). The chromosomes and signals were observed using an Olympus microscope BX61 equipped with a fluorescence lamp and the appropriate filters. Grey-scale images were captured using a DP70 cooled digital camera and were processed using Adobe Photoshop CS2 software.
Genome size estimation
The flow cytometric (FCM) analyses were conducted as described by Lopes et al. [54], in the Laboratory of Cytogenetics and Cytometry, Department of General Biology, Universidade Federal de Viçosa (UFV). The nuclear DNA contents of adult G. assimilis and E. surinamensis males and females were determined using the C DNA content of a Scaptotrigona xantotricha female as an internal standard, which was confirmed by comparison with that of the international standard, Drosophila melanogaster.
To prepare the nuclear suspensions for FCM, brain ganglia were excised from the standard animal and the sample animals in physiological saline solution (0.155 mM NaCl). The samples consisted of three males and three females of each species, and each individual was manipulated and analyzed separately, representing three independent repetitions. The materials were simultaneously crushed 10–12 times in a tissue grinder using a pestle (Kontes Glass Company, NJ, USA) in 100 μL of OTTO-I lysis buffer [55] containing 0.1 M citric acid (Merck, NJ, USA), 0.5% Tween 20 (Merck) and 50 μg mL-1 RNase A (Sigma-Aldrich), pH = 2.3. The suspensions were adjusted to 1.0 mL using the same buffer, filtered through a 30-μm nylon mesh (Partec, Nuremberg, Germany) and centrifuged at 100 g in microcentrifuge tubes for 5 min. The pellets were then incubated for 10–15 min in 100 μL of OTTO-I lysis buffer and were stained using 1.5 mL of OTTO-I:OTTO-II (1:2) solution [56,57] supplemented with 75 μM propidium iodide (PI) (excitation/emission wavelengths: 480-575/550-740 nm, [58]) and 50 μg mL-1 RNase A (Sigma-Aldrich), pH = 7.8. The nuclear suspensions were filtered through a 20-μm nylon mesh filter (Partec) and were maintained in the dark for 30 min.
The suspensions were analyzed using a Partec PAS flow cytometer (Partec) equipped with a laser source (488 nm). PI fluorescence emitted by the nuclei was collected using an RG 610-nm band-pass filter and converted to 1024 channels. The equipment was calibrated for linearity and was aligned using microbeads and standard solutions according to the manufacturer’s recommendations. FlowMax software (Partec) was used for data analysis. The standard nuclear peak was set to channel 100 and more than 10,000 nuclei were analyzed. Three independent replications were conducted, and histograms with a coefficient of variation (CV) of greater than 5% were rejected.
Results
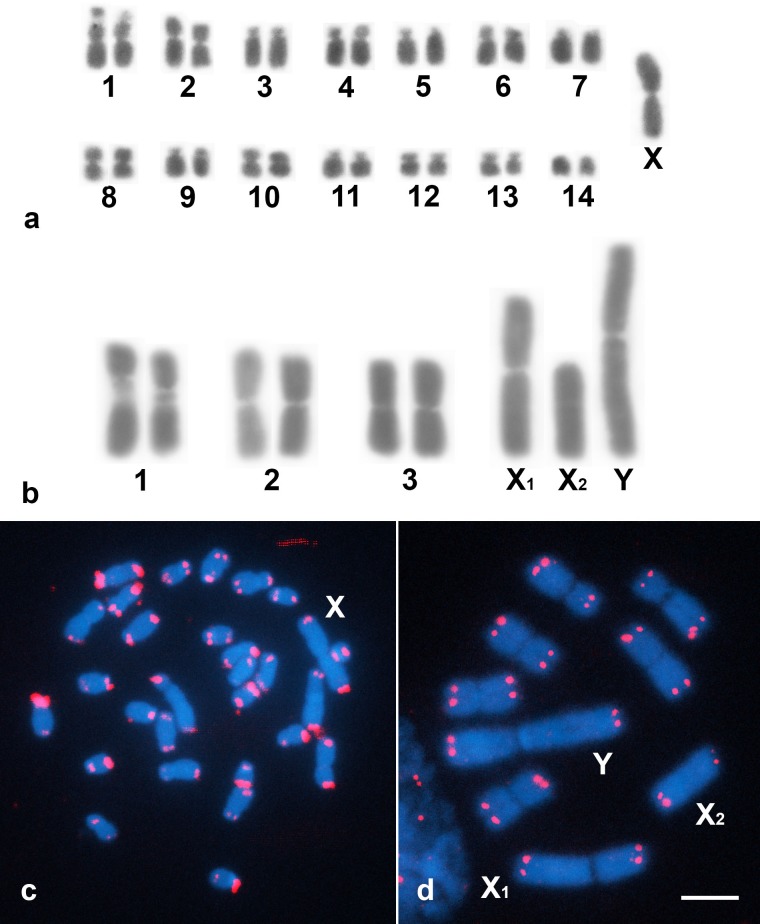
The karyotype of Gryllus assimilis is 2n = 29♂/30♀, with a X0♂/XX♀ sex-chromosome system (Fig 1A). The autosomes consist of four pairs of metacentric (1, 2, 8 and 10) chromosomes, four pairs of submetacentric (4, 6, 12 and 13) chromosomes, five pairs of subtelocentric (3, 5, 7, 9 and 11) chromosomes and one pair of telocentric (14) chromosomes. The X chromosome is metacentric and is the largest chromosome of the karyotype. One evident interstitial secondary constriction was observed in the metacentric pair of chromosome 1 (Fig 1A). Analysis of E. surinamensis males and females revealed a karyotype of 2n = 9♂/10♀ with a neo-X1X2Y♂/X1X1X2X2♀ sex-chromosome system. Autosomal pairs 2 and 3 are metacentric chromosomes, whereas pair 1 are submetacentric chromosomes with a conspicuous proximal secondary constriction localized on the long arm. The neo-X1 chromosome is metacentric, the neo-X2 chromosome is telocentric and the neo-Y chromosome, which is submetacentric, is the largest sex chromosome (Fig 1B). These results are similar to the previously described results for both species [12,40]. In both species, only terminal sites of the autosomes and sex chromosomes were recognized using the telomeric probe (Fig 1C and 1D).
Fig 1. Male karyotypes of Gryllus assimilis (a) and Eneoptera surinamensis (b) observed using Giemsa staining.
The chromosomes of both species were arranged in descending order of size. FISH analysis of the telomeric repeat in the mitotic cells of G. assimilis (c) and E. surinamensis (d). The sex chromosomes are indicated in the figure. Bar = 5 μm.
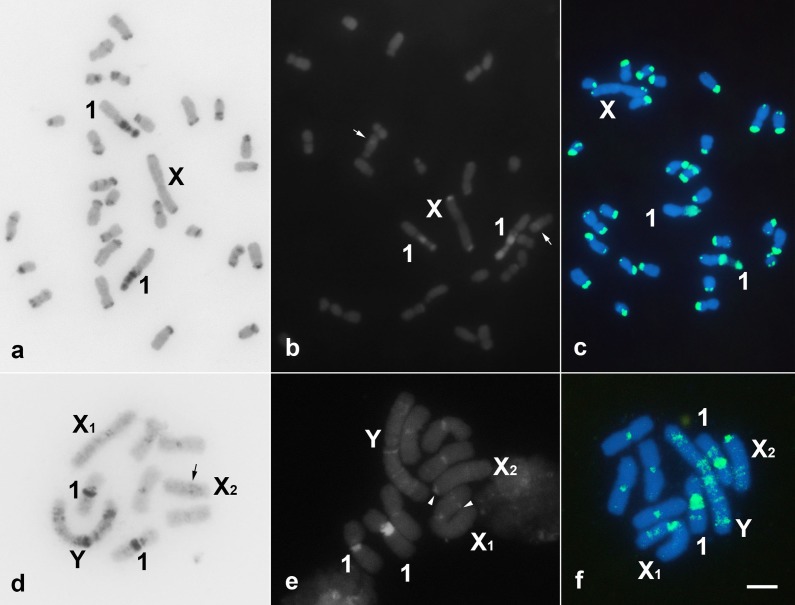
The C-positive regions were observed in distinct areas for the distinct chromosomes of G. assimilis, with more frequent terminal blocks on the short chromosomal arms and in the pericentromeric regions. The X chromosome harbored only C-positive terminal blocks in both arms. The secondary constriction of chromosomes 1 was intensely stained via C-banding (Fig 2A). In E. surinamensis, all of the chromosomes except the neo-X2 chromosome exhibited pericentromeric C-positive bands. The neo-X2 chromosome had a faint interstitial C-positive band, and the pair 1 chromosomes exhibited a heterochromatic block coincident with the secondary constriction. Additionally, some dispersed C-positive blocks were observed in the neo-Y chromosomes (Fig 2D). Most of the C-positive blocks in the G. assimilis chromosomes were neutral for G+C or A+T base pairs. A remarkable number of G+C positive blocks (CMA3 +) were observed only in the secondary constriction of the chromosome pair 1 and in the terminal region of the X chromosome, although faint CMA3 + signals were also observed in a few other chromosomes, in terminal or interstitial positions (Fig 2B). In the E. surinamensis chromosomes, all of the C-positive blocks were CMA3 + and the neo-X1 and neo-X2 chromosomes exhibited C-negative blocks rich in G+C base pairs, which were interstitial in the short arm and in the pericentromeric region, respectively (Fig 2E). No DAPI positive blocks were observed (result not shown). In both species, C-positive regions were also identified in the C 0 t-DNA fractions (Fig 2C and 2F). Additionally, in the E. surinamensis cells, faint signals were observed distributed along some of the chromosomes in the C-negative regions, which include the pericentromeric area of the neo-X2 chromosome (Fig 2F).
Fig 2. C-banding (a, d), CMA3 fluorochrome staining (b, e) and FISH of the C 0 t-DNA (c, f) on mitotic chromosomes of G. assimilis (a, b, c) and E. surinamensis (d, e, f) cells.
The sex chromosomes and the chromosomes bearing the secondary constrictions (pair 1) are indicated in each of the panels. Note the predominance of terminal C-positive blocks in G. assimilis and E. surinamensis chromosomes, the neo-Y chromosome with some heterochromatic blocks and CMA3 + signals along its entire length, as well as the C 0 t-DNA signals. In (b), the white arrow indicates the interstitial CMA3 + block, and in (d), the black arrow indicates the interstitial C-positive block in the neo-X2 chromosome. In (e) arrowheads indicate the CMA3 + signals in C-negative regions of the neo-X1 and neo-X2. One chromosome is missing in (b). Bar = 5 μm.
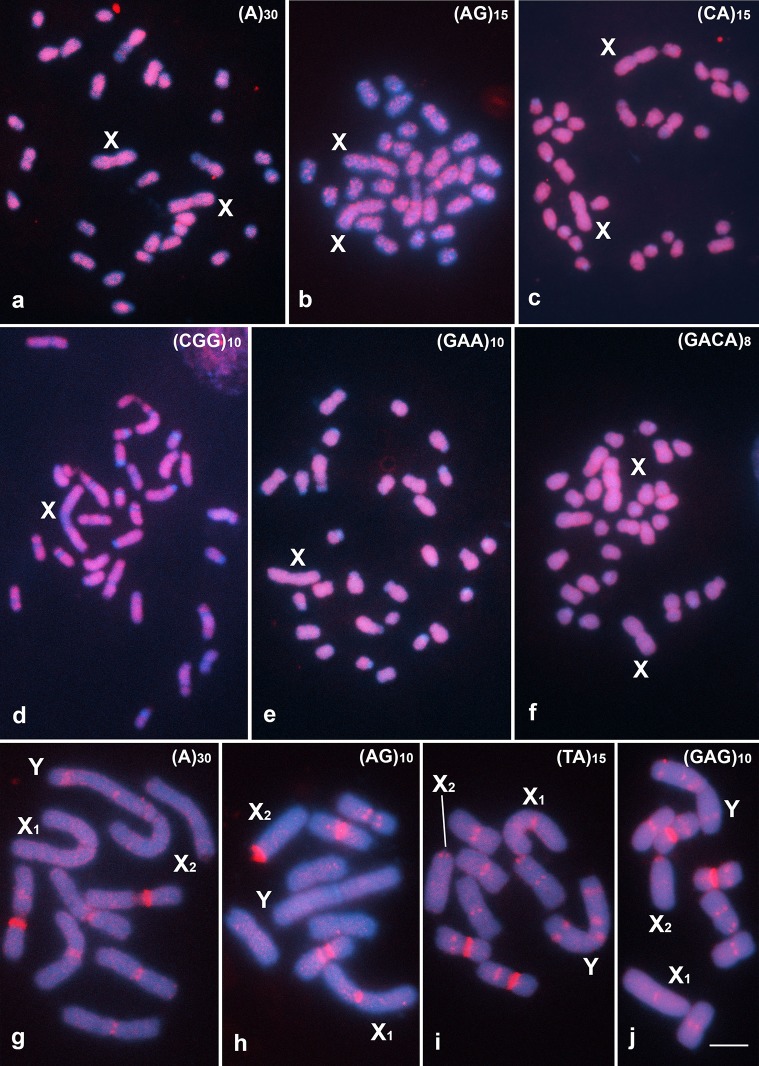
Labeling of the mono-, di-, tri and tetra-nucleotide arrays in G. assimilis cells revealed a relatively uniform dispersed distribution of each on all of the chromosomes (Fig 3A–3F). Only the (CGG)10 signal was less intense in the centromeric regions (Fig 3D). Although scattered signals for these arrays were generally observed in E. surinamensis cells, some of the signals formed band-like patterns in distinct chromosomes and in distinct positions, depending on the repeat mapped, with the exception of the (CAA)10 and (GATA)8 arrays, which showed only a scattered distribution (Fig 3G–3J, Table 1).
Fig 3. Mitotic metaphase cells of G. assimilis (a-f) and E. surinamensis (g-j), demonstrating examples of the hybridization patterns of different microsatellite-motif probes.
The sex chromosomes and the signals for each type of microsatellite motif are shown in the images. Note the intense signals along the chromosomal arms of both species, although band-like signals are also evident in distinct chromosomes and in distinct positions in E. surinamensis cells. One chromosome is missing in (b). Bar = 5 μm.
Table 1. Chromosomal positions of the band-like signals for microsatellite arrays in the cricket Eneoptera surinamensis.
| Microsatellite motif | Pair 1 | Pair 2 | Pair 3 | X1 | X2 | Y |
|---|---|---|---|---|---|---|
| (A)30 | S: p | c | c | L: i,sd | ||
| (C)30 | S: p | c | c | S: p,sd | ||
| (AG)10 | S: p; L: p | c | c | |||
| (CA)15 | S: p | c | c | L: i,sd | ||
| (CG)15 | c; L: 2i | c | c | c; L: 2p,i,sd; S: 2p,sd | ||
| (TA)15 | c; L: i | c,d | c | c | c | L: p,i,sd; S: sd |
| (CAC)10 | S: sd | L: 2p | ||||
| (CAG)10 | c | c | c | c | c | S: p; S: sd |
| (CGG)10 | c | c | c | c; L: p | ||
| (TAC)10 | c; L: 2i | c | c | c; L: 2p,sd; S: p,sd | ||
| (GAG)10 | c; L: i | c | c,d | c | c | L: p,i,sd; S: sd |
| (GAA)10 | c | c | c | S: p,sd | ||
| (TAA)10 | c | c | c | |||
| (GACA)4 | c; L: p | c | c | c; L: 2p,sd; S: p,sd |
L = long arm; S = short arm; c = centromeric; p = proximal; i = interstitial; sd = subdistal; d = distal.
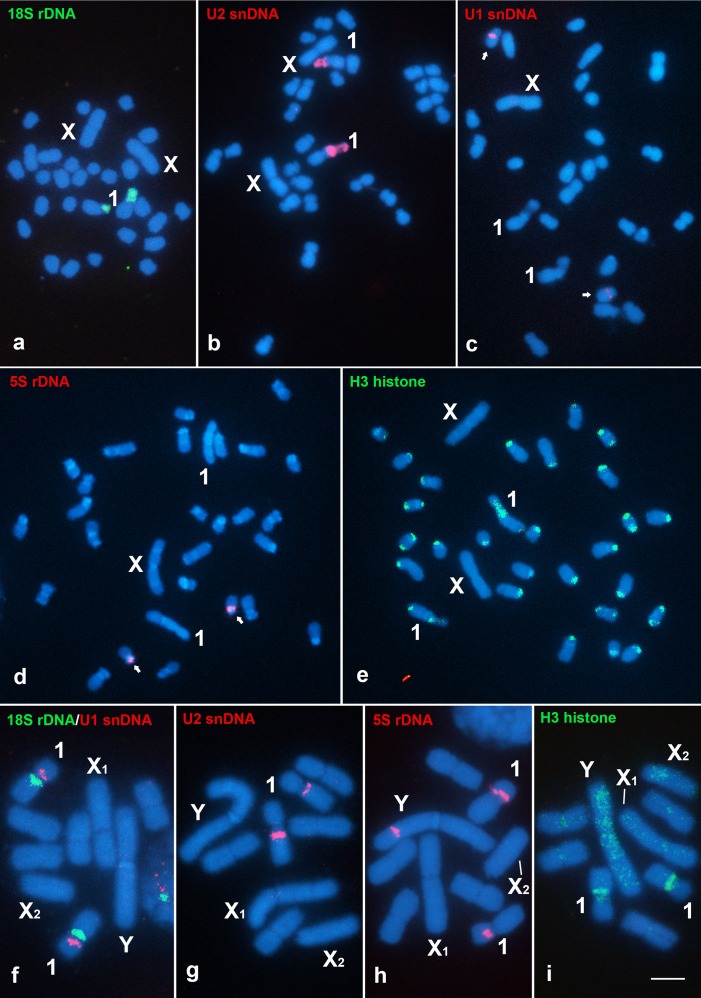
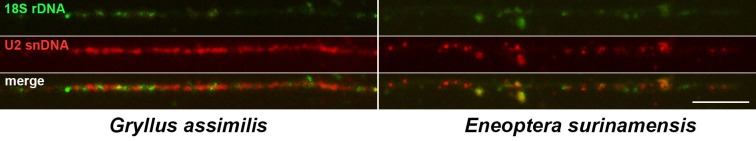
The FISH analyses revealed 18S rDNA and U2 snDNA signals on the pair 1 of G. assimilis, which were co-localized in the arm containing the secondary constriction (Fig 4A and 4B), whereas signals for 5S rDNA and U1 snDNA clusters were observed in the pericentromeric and proximal areas, respectively, of small subtelocentric chromosome pairs; however, due to slight differences in these chromosomes, it was not possible to precisely identify them (Fig 4C and 4D). The H3 histone signal occurred in evident bands in the terminal regions of some of the autosomes, whereas no H3 histone signal was observed on the X chromosome (Fig 4E). In E. surinamensis cells, labeling for all of the multigene families revealed clusters in the pair 1, and the 18S rDNA, U2 snDNA and H3 histone signals were coincident with the secondary constriction (Fig 4F, 4G and 4I), whereas the 5S rDNA and U1 snDNA signals were observed on the short arm (Fig 4F and 4H). However, an additional cluster of 5S rDNA labeling was observed in the interstitial region of the long arm of the neo-Y chromosome (Fig 4H), and a faint H3 histone signal was observed along all of the chromosomes, which was more evident in the neo-Y chromosome (Fig 4I). Fiber-FISH using probes for 18S rDNA and U2 snDNA, which were co-localized in the secondary constriction of the pair 1 chromosomes in metaphasic chromosomes in both species, revealed that these two sequences are interspersed in similar patterns (Fig 5).
Fig 4. Chromosomal mapping of multigene families in mitotic cells of G. assimilis (a-e) and E. surinamensis (f-i).
The sex chromosomes and the signals for each type of probe are shown in the images. Note the same chromosomal localization (pair 1) of 18S rDNA and U2 snDNA in both species and the signals for all of the multigene families in the pair 1 of E. surinamensis. Also, note the multiple and scattered signals of the H3 histone gene (e, i). The arrows in (c, d) indicate the chromosomes bearing signals. Bar = 5 μm.
Fig 5. Fiber-FISH of the 18S rDNA and U2 snDNA probes in G. assimilis (a) and E. surinamensis (b).
Note that these probes are co-localized and are interspersed in both species.
The FCM analyses revealed that the mean genome size (1C) of G. assimilis cells is 2.13 pg (male: 2.06 pg and female: 2.21 pg), whereas E. surinamensis presents approximately 2.5 times greater DNA content, with a mean value of 5.54 pg (male: 5.44 pg and female: 5.65 pg) (see S1 Fig). Thus, the mean genome size of G. assimilis is 2.08 Gb and that of E. surinamensis is 5.42 Gb. The greater size of the genomes of the females of both species is certainly due to the presence of two copies of the X chromosome in female G. assimilis cells and the presence of two copies for the X1 and X2 chromosomes in female E. surinamensis cells.
Discussion
Karyotypes and general organization of repetitive DNA
The two cricket species examined in this study have highly divergent karyotypes. Considering that fusions are more frequent in Orthopteran chromosomal evolutionary history (for example, see [8,9,59]), leading to a reduced diploid number, the karyotype of E. surinamensis could be considered more evolved relative to that of G. assimilis. Repetitive DNAs can be relevant markers for tracing the evolution of the karyotypes and genomes of eukaryotes, but there is little information concerning the sizes of cricket genomes or the organization of their repetitive DNAs and their possible role in chromosomal evolution [13,17,38,39].
The diploid number and the sex system of G. assimilis observed in this study is identical to that previously described [40] and resembles that of most of the other Gryllus species that have been analyzed [10,13,60,61,62]. However, reduction of the diploid number has been observed in this genus [10], which includes the polymorphic condition of G. assimilis [11]. The main difference observed here in relation to other Gryllus karyotypes is the chromosomal morphology and the chromosome bearing the evident secondary constriction (see above references), suggesting that although Gryllus exhibits great karyotypic stability, small structural rearrangements may have occurred during the evolution of the genus. The diploid number observed in E. surinamensis (2n = 9) is well established in this species, having been observed in certain populations [63], and considering that a higher number of diploid chromosomes is more common in Gryllidae crickets [9,12,64], the karyotype of this species appears to be highly rearranged. Autosomal and autosomal/sex chromosome reshuffling most likely caused the significant reduction in the diploid number and the origin of a multiple sex system (neo-X1X2Y) with large chromosomes. It is difficult to specify the evolutionary causes or the order of the specific chromosomal rearrangements that led to the origin of the extreme karyotype of E. surinamensis, due to its extensive reorganization and the scarce phylogenetic and chromosomal information available for crickets. However, Robertsonian translocations (Rb-translocation), tandem fusions and pericentric inversions are most likely involved. In all cases of Rb-translocations or tandem fusions, the telomeres appeared to be either lost during the chromosomal rearrangement or eliminated during chromosome differentiation and no internal telomere sites are observed, as indicated by our FISH-based mapping of the telomere motif, which revealed signals only in the actual telomeres. A similar condition was reported in a small number of studied grasshoppers with derived karyotypes in which centric fusion or Rb-translocation occurred [15,16]. However, due to the limited sensitivity of the classical FISH technique, the possibility of small internal telomeric sites cannot be completely ruled out. In Gryllus, if the karyotype experienced inversions, as justified by distinct chromosomal morphologies relative to those of other species, these rearrangements occurred without the involvement of telomeres or the telomeres were lost. An alternative explanation for this condition is that G. assimilis has a non-rearranged karyotype compared with those of other species of the genus, which should be evaluated using a better species sampling.
There is little information concerning heterochromatin distribution in crickets, but in the genus Gryllus, the occurrence of mainly centromeric and terminal heterochromatic blocks has been observed in certain species, i.e., G. bimaculatus, G. argentinus and Gryllus sp. [13,60,62], as observed in this study in G. assimilis. In contrast, in E. surinamensis, the C-positive blocks were most organized in the centromeric region of the autosomes, as well as in other chromosomal regions, with extensive spreading in the neo-Y chromosome. These patterns, including those observed in Cycloptiloides americanus [17], suggest the intense reorganization of the C-positive blocks in the rearranged karyotypes. These data indicate that the heterochromatin chromosomal dynamic is more intense in crickets than in other Orthoptera, such as grasshoppers, in which pericentromeric C-positive blocks are most commonly observed [65,66], although other species should be studied. The data obtained through fluorochrome staining suggests the occurrence of repetitive DNA families with distinct richness in A+T or G+C base pairs, which in E. surinamensis are G+C-rich, whereas in G. assimilis, they are mainly neutral for A+T or G+C, suggesting the restructuring of repetitive DNA families in their two karyotypes. This data was confirmed in E. surinamensis through genomic analysis that revealed the occurrence of mainly G+C-rich satDNAs (Palacios-Gimenez et al., unpublished data). In another Gyllus species, i.e., G. bimaculatus, some terminal heterochromatic blocks were enriched for two A+T-rich satDNAs families, one of which is also present in other Gryllus species, including G. rubens and Gryllus sp [13]. The occurrence of A+T-rich repetitive families in the genome of G. assimilis could not be ruled out, and DAPI+ blocks could not be observed in this organism due to the limits of resolution.
The chromosomal organization of repetitive DNAs obtained through C-banding was corroborated by C 0 t-DNA mapping that revealed a similar pattern, as well as additional regions enriched in repetitive sequences, such as the centromere of the neo-X1 chromosome of E. surinamensis and some faintly labelled dispersed areas. In other Orthopteran species, the C 0 t-DNA fraction is also enriched in C-positive autosomal blocks, but divergent patterns have been observed in the sex chromosomes, including the derivate neo-sex chromosomes, as observed in E. surinamensis. For example, enrichment of repetitive DNAs in the neo-Y element of the grasshopper Ronderosia bergi [16] and the X2 element of the cricket C. americanus [17] have been reported, suggesting a role of this genomic fraction in sex chromosome differentiation, causing chromatin to change into heterochromatin. The accumulation of repetitive DNAs is a common feature during the differentiation of the Y or W sex chromosomes, and it has been documented in various animal and plant species, such as Rumex species [27,67], Silene latifolia [30], lizard species [68], Schistosoma mansoni [69] and Drosophila miranda [70,71]. However, in some grasshopper species, the C 0 t-DNA fraction is restricted to the centromeres of the sex chromosomes [15].
The FISH mapping results regarding the microsatellites suggest that a major strong differentiation in the chromosomal organization of the karyotypes of the two species occurred. Although the repeats are mainly dispersed in both euchromatin and heterochromatin, clustering was observed, with the repeats forming a band-like pattern, particularly those in the heterochromatic regions of the pair 1 and sex chromosomes of E. surinamensis. This result suggests that the reduction in the diploid number was followed by the “compartmentalization” of the microsatellites in the genome, with these sequences being involved in the heterochromatin, including those of the sex chromosomes. The accumulation of some of the microsatellite arrays in the neo-Y chromosomes of E. surinamensis was expected, considering that they are non-recombining elements, as suggested by the lack of contact of these three chromosomes during meiosis [8], and this data is consistent with that observed in other species [72,73,74,75]. As previously observed in the grasshopper R. bergi, the microsatellites of E. surinamensis are clearly involved in the differentiation between sex chromosomes [16]. Although poorly studied in Orthoptera, the dispersal or specific chromosomal distribution of the microsatellite arrays in band-like patterns was recently reported in other species, such as the cricket C. americanus [17] and the grasshoppers Abracris flavolineata [76], R. bergi [16], Locusta migratoria and Eyprepocnemis plorans [77]. However, next generation sequencing analysis revealed uneven and nonrandom localization of microsatellites in L. migratoria and E. plorans, with the dinucleotide motifs predominantly associated with other repetitive DNAs, such as the histone gene spacer, rDNA intergenic spacers (IGSs) and transposable elements [77]. A similar pattern was observed in E. surinamensis, in which some of the microsatellite arrays that are distributed in a band-like pattern are co-localized with some of the multigene families and the anonymous C 0 t-DNA fraction.
The increased size of the genome of E. surinamensis compared with that of G. assimilis is notable, and considering the little information available concerning the size of the genomes of crickets [38,39], this is apparently a derivate characteristic. The increased size of this genome could be attributed to the amplification, spreading and accumulation of repetitive DNAs in specific chromosomal regions, such as observed for C 0 t-DNA and some satellite repeats (Palacios-Gimenez et al., unpublished data), besides some microsatellites. Although, there is no evidence that the reduction in the diploid number contributed to this process. This process is more evident in the derivate sex chromosomes, most likely due to the non-recombinant state of the neo-Y chromosome and the restricted recombination of the neo-X1 and neo-X2 chromosomes in the female germline. Additionally, the structural mutation rates of the neo-Y chromosomes of some species are more rapid than those of other genomic regions, suggesting the reduced efficacy of natural selection in this chromosome [18,78,79,80], which is likely to occur in the genome of E. surinamensis.
The multigene families
No variation in the location and number of clusters of 18S rDNA and U2 snDNA was observed, both of which are conserved on the pair 1. In grasshoppers [81,82] and other insects, such as Lepidoptera [83], Coleoptera [84] and Heteroptera [85], extremely variable patterns of the major rDNA was observed contrasting with this study. Remarkably, the pattern of U2 snDNA on the pair 1 is highly conserved in some grasshopper species [15,48] and in the cricket C. americanus (Mogoplistidae) [17]. Conservation of the number of U2 snDNA clusters is observed also in other groups, such as Gymnotus species [86], in contrast to the case in some of the other fish species of the Batrachoididae family, in which the U2 snDNA signals appear to be widely scattered [87]. The 18S rDNA and U2 snDNA signals were located in the same sites and exhibited the same association in the two species, which was supported by the fiber-FISH results, which may indicate an ancestral characteristic in Gryllidae that was maintained despite the exceptional chromosomal divergence. However, other species in the family should be studied to confirm this hypothesis. The association or interspersion of distinct multigene families has been reported in distinct groups, but the significance of these patterns is not clear and they have no apparent selective advantage [45,82,84,88,89,90,91].
The presence of 5S rDNA and U1 snDNA in the small chromosomes of G. assimilis in contrast to their presence in the pair 1 of E. surinamensis may be the consequence of large-scale chromosomal rearrangements, as mentioned above, which translocated these genes to the same chromosome. However, the localization of 5S rDNA in the neo-Y chromosome of E. surinamensis suggests both amplification and transposition or even further chromosomal rearrangement involving sex chromosomes and ancestral autosomes bearing this gene, as has been suggested for other Orthopteran species that have neo-sex chromosomes [15,16,17]. The presence of this gene in the neo-Y chromosomes and absence in the neo-X chromosomes reinforce the differentiation between the neo-sex chromosomes of E. surinamensis that was established after the chromosomal rearrangements occurred.
Contrary to what was observed for rDNAs and U snDNAs, significant dispersion of the H3 histone gene occurred in G. assimilis and E. surinamensis, with differentially spread patterns observed in the two species, with these DNA elements clearly in blocks in G. assimilis and faint signals observed in E. surinamensis. These results suggest the independent amplification/dispersion and dynamism of the H3 histone genes at the chromosomal level. Multiple H3 histone sites, in blocks such as observed in G. assimilis, have rarely been reported in grasshoppers, such as Abracris flavolineata [48], Dichromatos lilloanus [15] and Ronderosia bergi [16]. Although, this pattern is less common, being commonly observed an interstitial cluster in the pair 8 a highly conserved character [92]. Our data suggest that the organization and evolution of the H3 histone genes may be more dynamic than was previously reported and that this dispersal in G. assimilis and E. surinamensis could be due to other mechanisms, such as association with other repetitive DNAs (transposons or satDNAs), ectopic recombination or extrachromosomal circular DNA (eccDNA), as has been proposed for rDNAs [19,81,93]. An alternative hypothesis for the dispersal of H3 histone genes is the similarity of the sequences of these genes with those of unknown repetitive DNAs. Remarkably, a main cluster of H3 histone genes occurs in the pair 1, as is the case for the other four multigene families investigated in this study. This arrangement of certain multigene families in one unique bivalent chromosome has not yet been described and could be result of the large-scale rearrangements observed in the E. surinamensis karyotype.
In conclusion, our study provided an opportunity to explore the evolutionary dynamics of repetitive DNAs in two non-model species and its possible involvement in karyotypic organization and genome increasing. As we suggested some of the differences between the two evaluated karyotypes were most likely a consequence of the evolution and distribution of repetitive DNA that were accumulated in specific chromosomal regions. Whereas certain characteristics that these species shared could indicate their ancestral relationship. Moreover, the distinct patterns of the classes of repetitive sequences that were mapped in this study suggests that the differential amplification and accumulation of this class of DNA occurred mainly in the neo-Y chromosomes, highlighting the differentiation of the neo-sex chromosomes of E. surinamensis.
Supporting Information
(PDF)
Acknowledgments
The authors are grateful to the “Parque Estadual Edmundo Navarro de Andrade” administration for sample collecting authorization, to Antonio Sergio Pascon for technical assistance in obtaining embryos and to Edison Zefa and Luciano Martins for the taxonomic identification of Gryllus assimilis. OMPG acknowledges the scholarship obtained from FAPESP (process number 2014/02038-8). DCCM was the recipient of a research productivity fellowship from the Conselho Nacional de Desenvolvimento Científico e Tecnológico-CNPq (process number 304758/2014-0).
Abbreviations
- 2n
diploid number
- C0t-DNA
C 0 is the initial concentration of single-stranded DNA in mol/l and t is the reannealing time in seconds
- CMA3
Chromomycin A3
- DA
Distamicyn
- DAPI
4’, 6-Diamidino-2-phenylindole
- FCM
Flow cytometry
- FISH
Fluorescence in situ hybridization
- PCR
Polymerase chain reaction
- PI
Propidium iodide
- Rb-translocation
Robertsonian translocation
- rDNA
Ribosomal DNA
- rRNA
Ribosomal RNA
- snRNA
Small nuclear RNA
Data Availability
The data underlying the findings described in the paper is freely available to other researchers (1) in the body of the paper and (2) in the supporting information.
Funding Statement
This study was funded by a scholarship obtained from the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, process numbers 2014/11763-8, 2014/02038-8) and a research productivity fellowship from the Conselho Nacional de Desenvolvimento Científico e Tecnológico-CNPq (CNPQ, process number 304758/2014-0). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Grimaldi D, Engel MS. Evolution of the insects Cambridge University Press, New York; 2005. [Google Scholar]
- 2.Eades DC, Otte D, Cigliano MM, Braun H. Orthoptera species file. Version 5.0/5.0. [21/6/2015]. Availible: http://Orthoptera.speciesfile.org.
- 3. Song H, Amédégnato C, Cigliano MM, Desutter-Grandcolas L, Heads SW, Huang Y, et al. 300 million years of diversification: elucidating the patterns of orthopteran evolution based on comprehensive taxon and gene sampling. Cladistics. 2015. February 4 10.1111/cla.12116 [DOI] [PubMed] [Google Scholar]
- 4. Warchalowska-Śliwa E. Karyotype characteristics of katydid Orthopterans (Ensifera, Tettigoniidae) and remarks on their evolution at different taxonomic levels. Folia Biol. 1998;4: 143–176. [Google Scholar]
- 5. Ferreira A, Mesa A. Cytogenetics studies in Brazilian species of Pseudophyllinae (Orthoptera: Tettigoniidae): 2n(♂) = 35 and FN = 35 the probable basic and ancestral karyotype of the family Tettigoniidae. Neotr Entomol. 2010;39: 590–594. [DOI] [PubMed] [Google Scholar]
- 6. Grzywacz B, Heller KG, Lehmann AW, Warchałowska-Śliwa E, Lehmann GUC. Chromosomal diversification in the flightless Western Mediterranean bush cricket genus Odontura (Orthoptera: Tettigoniidae: Phaneropterinae) inferred from molecular data. J Zoolog Syst Evol Res. 2014;52: 109–118. [Google Scholar]
- 7. Sáez FA. An extreme karyotype in an orthopteran insect. Amer Nat. 1957;91: 259–264. [Google Scholar]
- 8. White MD. Animal cytology and evolution 3re ed. Cambridge Univ. Press, London; 1973. [Google Scholar]
- 9. Hewitt GM. Grasshoppers and crickets Animal cytogenetics. vol 3: Insecta 1. Orthoptera.Gebrüder Borntraeger, Berlin; 1979. [Google Scholar]
- 10. Handa SM, Mittal OP, Sehgal S. Cytology of ten species of crickets from Chandigarh (lndia). Cytologia. 1985;50: 711–72. [Google Scholar]
- 11. Zefa E. Autosomal rearrangement in Gryllus assimilis Fabricius, 1775 (Orthoptera, Gryllidae). Genet Mol Biol. 1999;22: 333–336. [Google Scholar]
- 12. Ferreira A, Cella DM. Chromosome structure of Eneoptera surinamensis (Orthoptera, Grilloidea, Eneopterinae) as revealed by C, NOR and N-banding techniques. Chromosome Sci. 2006;9: 47–51. [Google Scholar]
- 13. Yoshimura A, Nakata A, Mito T, Noji S. The characteristics of karyotype and telomeric satellite DNA sequences in the cricket, Gryllus bimaculatus (Orthoptera, Gryllidae). Cytogenet. Genome Res. 2006;112: 329–336. [DOI] [PubMed] [Google Scholar]
- 14. Castillo ER, Martí DA, Bidau CJ. Sex and neo-sex chromosomes in Orthoptera: a review. J Orthopt Res. 2010;19: 213–231. [Google Scholar]
- 15. Palacios-Gimenez OM, Castillo ER, Martí DA, Cabral-de-Mello DC. Tracking the evolution of sex chromosome systems in Melanoplinae grasshoppers through chromosomal mapping of repetitive DNA sequences. BMC Evol Biol. 2013;13: 167 10.1186/1471-2148-13-167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Palacios-Gimenez OM, Marti DA, Cabral-de-Mello DC. Neo-sex chromosomes of Ronderosia bergi: insight into the evolution of sex chromosomes in grasshoppers. Chromosoma. 2015. January 21 10.1007/s00412-015-0505-1 [DOI] [PubMed] [Google Scholar]
- 17. Palacios-Gimenez OM, Cabral-de-Mello DC. Repetitive DNA chromosomal organization in the cricket Cycloptiloides americanus: a case of the unusual X1X20 sex chromosome system in Orthoptera. Mol Genet Genomics. 2015;290: 623–631. 10.1007/s00438-014-0947-9 [DOI] [PubMed] [Google Scholar]
- 18. Charlesworth B, Sniegowski P, Stephan W. The evolutionary dynamics of repetitive DNA in eukaryotes. Nature. 1994;371: 215–220. [DOI] [PubMed] [Google Scholar]
- 19. Nei M, Rooney AP. Concerted and birth-and-death evolution of multigene families. Annu Rev Genet. 2005;39: 121–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Biémont C, Vieira C. Genetics: junk DNA as an evolutionary force. Nature. 2006;443: 521–524. [DOI] [PubMed] [Google Scholar]
- 21. Richard GF, Kerrest A, Dujon B. Comparative genomics and molecular dynamics of DNA repeats in eukaryotes. Microbiol Mol Biol Rev. 2008;72: 686–727. 10.1128/MMBR.00011-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. López-Flores I, Garrido-Ramos MA. The repetitive DNA content of eukaryotic genomes In: Garrido-Ramos MA editor. Repetitive DNA. Genome Dyn. Basel, Karger, vol 7; 2012. pp. 1–28. 10.1159/000337118 [DOI] [PubMed] [Google Scholar]
- 23. Doolittle WF, Sapienza C. Selfish genes, the phenotype paradigm and genome evolution. Nature. 190;284: 601–603. [DOI] [PubMed] [Google Scholar]
- 24. Orgel LE, Crick FHC. Selfish DNA: the ultimate parasite. Nature. 1980;284: 604–607. [DOI] [PubMed] [Google Scholar]
- 25. Charlesworth B. The evolution of chromosomal sex determination and dosage compensation. Curr Biol. 1996;6: 149–162. [DOI] [PubMed] [Google Scholar]
- 26. Skaletsky H, Kuroda-Kawaguchi T, Minx PJ, Cordum HS, Hillier L, Brown LG, et al. The male-specific region of the human Y chromosome is a mosaic of discrete sequence classes. Nature. 2003;423: 825–837. [DOI] [PubMed] [Google Scholar]
- 27. Navajas-Pérez R, de la Herrán R, Jamilena M, Lozano R, Ruiz Rejón C, Ruiz Rejón M, Garrido-Ramos MA (2005). Reduced rates of sequence evolution of Y-linked satellite DNA in Rumex (Polygonaceae). J Mol Evol. 2005;60: 391–399. [DOI] [PubMed] [Google Scholar]
- 28. Eickbush TH, Eickbush DG. Finely orchestrated movements: evolution of the ribosomal RNA genes. Genetics. 2007;175: 477–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Matsunaga S. Junk DNA promotes sex chromosome evolution. Heredity. 2009;102: 525–526. 10.1038/hdy.2009.36 [DOI] [PubMed] [Google Scholar]
- 30. Kejnovsky E, Hobza R, Cermak T, Kubat Z, Vyskot B. The role of repetitive DNA in structure and evolution of sex chromosomes in plants. Heredity. 2009;102: 533–541. 10.1038/hdy.2009.17 [DOI] [PubMed] [Google Scholar]
- 31. Kidwell MG, Lisch D. Transposable elements as sources of variation in animals and plants. Proc Natl Acad Sci USA. 1997;94: 7704–7711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gregory TR. The evolution of the genome Elsevier Academic Press, San Diego CA USA; 2005. [Google Scholar]
- 33. Lynch M. The origins of genome architecture Sinauer Associates, Inc. Publishers, Sunderland, MA, USA; 2007. [Google Scholar]
- 34. Rasch EM, Rasch RW. Cytophotometric determination of genome size for two species of cave crickets (Orthoptera, Rhaphidophoridae). J Histochem Cytochem. 1991;29: 885. [Google Scholar]
- 35. Westerman M, Barton NH, Hewitt GM. Differences in DNA content between two chromosomal races of the grasshopper Podisma pedestris . Heredity. 1987;58: 221–228. [Google Scholar]
- 36. Wang X, Fang X, Yang P, Jiang X, Jiang F, Zhao D, et al. The locust genome provides insight into swarm formation and long-distance flight. Nature Commun. 2014;5: 2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Camacho JPM, Ruiz-Ruano FJ, Martín-Blázquez R, López-León MD, Cabrero J, Lorite P, et al. A step to the gigantic genome of the desert locust: chromosome sizes and repeated DNAs. Chromosoma. 2015;124: 263–275. 10.1007/s00412-014-0499-0 [DOI] [PubMed] [Google Scholar]
- 38. Hanrahan SJ, Johnston S. New genome size estimates of 134 species of arthropods. Chromosome Res. 2011;19: 809–823. 10.1007/s10577-011-9231-6 [DOI] [PubMed] [Google Scholar]
- 39.Gregory TR. Animal Genome Size Database. 2015. Available: http://www.genomesize.com.
- 40. Baumgartner WJ. Some evidence for the individuality of the chromosomes. Biol Bull. 1904;8: 1–23. [Google Scholar]
- 41. Webb GC, White MJD, Contreras N, Cheney J. Cytogenetics of the parthogenetic grasshopper Warramaba (formerly Moraba) virgo and its bisexual relatives. IV. Chromosome banding studies. Chromosoma. 1978;67: 309–339. [Google Scholar]
- 42. Sumner AT. A simple technique for demonstrating centromeric heterochromatin. Exp Cell Res. 1972;75: 304–306. [DOI] [PubMed] [Google Scholar]
- 43. Schweizer D, Mendelak M, White MJD, Contreras N. Cytogenetics of the parthenogenetic grasshopper Warramaba virgo and its bisexual relatives. X. Pattern of fluorescent banding. Chromosoma. 1983;88: 227–236. [Google Scholar]
- 44. Sambrook J, Russel DW. Molecular cloning: A laboratory manual Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2001. [Google Scholar]
- 45. Cabral-de-Mello DC, Moura RC, Martins C. Chromosomal mapping of repetitive DNAs in the beetle Dichotomius geminatus provides the first evidence for an association of 5S rRNA and histone H3 genes in insects, and repetitive DNA similarity between the B chromosome and A complement. Heredity. 2010;104: 393–400. 10.1038/hdy.2009.126 [DOI] [PubMed] [Google Scholar]
- 46. Colgan DJ, McLauchlan A, Wilson GDF, Livingston SP, Edgecombe GD, Macaranas J, et al. Histone H3 and U2 snRNA DNA sequences and arthropod molecular evolution. Austral J Zool. 1998;46: 419–437. [Google Scholar]
- 47. Cabral-de-Mello DC, Valente GT, Nakajima RT, Martins C. Genomic organization and comparative chromosome mapping of the U1 snRNA gene in cichlid fish, with an emphasis in Oreochromis niloticus . Chromosome Res. 2012;20: 279–292. 10.1007/s10577-011-9271-y [DOI] [PubMed] [Google Scholar]
- 48. Bueno D, Palacios-Gimenez OM, Cabral-de-Mello DC. Chromosomal mapping of repetitive DNAs in Abracris flavolineata reveal possible ancestry for the B chromosome and surprisingly H3 histone spreading. PLoS ONE. 2103;8: e66532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zwick MS, Hanson RE, McKnight TD, Nurul-Islam-Faridi M, Stelly DM. A rapid procedure for the isolation of C 0 t–1 DNA from plants. Genome. 1997;40: 138–142. [DOI] [PubMed] [Google Scholar]
- 50. Ijdo JW, Wells RA, Baldini A, Reeders ST. Improved telomere detection using a telomere repeat probe (TTAGGG)n generated by PCR. Nucleic Acids Res. 1991;19: 4780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Pinkel D, Straume T, Gray JW. Cytogenetic analysis using quantitative, high sensitivity, fluorescence hybridization. Proc Natl Acad Sci USA. 1986;83: 2934–2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. de Barros AV, Sczepanski TS, Cabrero J, Camacho JPM, Vicari MR, Artoni RF. Fiber FISH reveals different patterns of high-resolution physical mapping for repetitive DNA in fish. Aquaculture. 2011;322: 47–50. [Google Scholar]
- 53. Camacho JPM, Cabrero J, López-León MA, Cabral-de-Mello DC, Ruiz-Ruano FJ. Grasshoppers (Orthoptera) In: Sharakhov V editor. Protocols for cytogenetic mapping of arthropod genomes. Boca Ratton, FL, USA: CRC Press; 2015. pp. 381–438. [Google Scholar]
- 54. Lopes DM, Carvalho CR, Clarindo WR, Praça MM, Tavares MG. Genome size estimation of three stingless bee species (Hymenoptera, Meliponinae) by flow cytometry. Apidologie. 2009;40: 517–523. [Google Scholar]
- 55.Otto F. DAPI staining of fixed cells for high-resolution flow cytometry of nuclear DNA. In: Darzynkiewicz Z, Crissman HA editors. Methods in cell biology. Vol 33; 1990. pp. 105–110. [DOI] [PubMed]
- 56. Loureiro J, Rodriguez E, Doležel J, Santos C. Comparison of four nuclear isolation buffers for plant DNA flow cytometry. Ann Bot. 2006;98: 679–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Loureiro J, Rodriguez E, Doležel J, Santos C. Flow cytometric and microscopic analysis of the effect of tannic acid on plant nuclei and estimation of DNA content. Ann Bot. 2006;98: 515–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Shapiro HM. Practical flow cytometry, 4th ed., John Wiley & Sons, New Jersey; 2003. [Google Scholar]
- 59. Mesa A, Ferreira A, Carbonell CS. Cariologia de los acridios neotropicales: estado actual de su conocimiento y nuevas contribuciones. Ann Soc Entomol Fr (NS). 1982; 18: 507–526. [Google Scholar]
- 60. Drets ME, Stoll M. C-banding and non-homologous associations in Gryllus argentinus . Chromosoma. 1974;48: 367–390. [DOI] [PubMed] [Google Scholar]
- 61. Lamborot M, Alvarez-Sarret E. The chromosomes of Gryllus field cricket populations in central Chile (Insecta: Grylloptera: Gryllidae).Can J Zoolog. 1985;63: 2626–2631. [Google Scholar]
- 62. Yoshimura A. Karyotypes of two American field crickets, Gryllus rubens and Gryllus sp. (Orthoptera: Gryllidae). Entomol Sci. 2005;8: 219–222. [Google Scholar]
- 63. Zefa E, Cordeiro J, Blauth M, Piumbini M, Silva AF, Costa MKM, Martins LP. Expanding the geographic cytogenetic studies in the bush crickets Eneoptera surinamensis (De Geer, 1773) (Orthoptera, Gryllidae, Eneopterinae) from Brazilian Atlantic and Amazon Forest. Zootaxa. 2014;3860: 396–400. 10.11646/zootaxa.3860.4.7 [DOI] [PubMed] [Google Scholar]
- 64. Mesa A, Bran EJ. Acerca de los cromosomas de Eneoptera surinamensis . An II Cong Latino-Americ Zol S Paulo. 1964;I: 9–16. [Google Scholar]
- 65. Santos JL, Arana P, Giráldez L. Chromosome C-banding patterns in Spanish Acridoidea. Genetica. 1983;61: 65–74. [Google Scholar]
- 66. John B, King M. The inter-relationship between heterochromatin distribution and chiasma distribution. Genetica. 1985; 66: 183–194. [Google Scholar]
- 67. Steflova P, Tokan V, Vogel I, Lexa M, Macas J, Novak P, et al. Contrasting patterns of transposable element and satellite distribution on sex chromosomes (XY1Y2) in the dioecious plant Rumex acetosa . Genome Biol Evol. 2013; 5: 769–782. 10.1093/gbe/evt049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Pokorná M, Kratochvíl L, Kejnovský E. Microsatellite distribution on sex chromosomes at different stages of heteromorphism and heterochromatinization in two lizard species (Squamata: Eublepharidae: Coleonyx elegans and Lacertidae: Eremias velox). BMC Genet. 2011;12: 90 10.1186/1471-2156-12-90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Lepesant JMJ, Cosseau C, Boissier J, Freitag M, Portela J, Climent D, et al. Chromatin structural changes around satellite repeats on the female sex chromosome in Schistosoma mansoni and their possible role in sex chromosome emergence. Genome Biol. 2012;13: R14 10.1186/gb-2012-13-2-r14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Steinemann S, Steinemann M. The enigma of Y chromosome degeneration: TRAM,a novel retrotransposonis preferentially located on the Neo-Y chromosome of Drosophila miranda . Genetics. 1997;145: 261–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Steinemann S, Steinemann M. Retroelements: tools for sex chromosome evolution. Cytogenet Genome Res. 2005;110: 134–143. [DOI] [PubMed] [Google Scholar]
- 72. Kubat Z, Hobza R, Vyskot B, Kejnovsky E. Microsatellite accumulation on the Y chromosome in Silene latifolia . Genome. 2008;51: 350–356. 10.1139/G08-024 [DOI] [PubMed] [Google Scholar]
- 73. Kejnovský E, Michalovova M, Steflova P, Kejnovska I, Manzano S, Hobza R, et al. Expansion of microsatellites on evolutionary young Y chromosome. PLoS One. 2013;8: e45519 10.1371/journal.pone.0045519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Matsubara K, Knopp T, Sarre SD, Georges A, Ezaz T. Karyotypic analysis and FISH mapping of microsatellite motifs reveal highly differentiated XX/XY sex chromosomes in the pink-tailed worm-lizard (Aprasia parapulchella, Pygopodidae, Squamata). Mol Cytogenet. 2013;6: 60 10.1186/1755-8166-6-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Matsubara K, O'Meally D, Azad B, Georges A, Sarre SD, Graves JA, et al. Amplification of microsatellite repeat motifs is associated with the evolutionary differentiation and heterochromatinization of sex chromosomes in Sauropsida. Chromosoma. 2015. July 21 10.1007/s00412-015-0531-z [DOI] [PubMed] [Google Scholar]
- 76. Milani D, Cabral-de-Mello DC. Microsatellite organization in the grasshopper Abracris flavolineata (Orthoptera: Acrididae) revealed by FISH mapping: remarkable spreading in the A and B chromosomes. PLoS ONE. 2014;9: e97956 10.1371/journal.pone.0097956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Ruiz-Ruano FJ, Cuadrado Á, Montiel EE, Camacho JPM, López-León MD. Next generation sequencing and FISH reveal uneven and nonrandom microsatellite distribution in two grasshopper genomes. Chromosoma. 2015;124: 221–234. 10.1007/s00412-014-0492-7 [DOI] [PubMed] [Google Scholar]
- 78. Clark AG. The vital Y chromosome. Nature. 2014;508: 463–465. 10.1038/508463a [DOI] [PubMed] [Google Scholar]
- 79. Toups M, Veltsos P, Pannell JR. Plant sex chromosomes: lost genes with little compensation. Curr Biol. 2015;25: 427–429. [DOI] [PubMed] [Google Scholar]
- 80. Bergero R, Qiu S, Charlesworth D. Gene loss from a plant sex chromosome system. Curr Biol. 2015;25: 1234–1240. 10.1016/j.cub.2015.03.015 [DOI] [PubMed] [Google Scholar]
- 81. Cabrero J, Camacho JP. Location and expression of ribosomal RNA genes in grasshoppers: abundance of silent and cryptic loci. Chromosome Res. 2008;16: 595–607. 10.1007/s10577-008-1214-x [DOI] [PubMed] [Google Scholar]
- 82. Cabral-de-Mello DC, Cabrero J, López-León MD, Camacho JPM. Evolutionary dynamics of 5S rDNA location in acridid grasshoppers and its relationship with H3 histone gene and 45S rDNA location. Genetica. 2011;139: 921–931. 10.1007/s10709-011-9596-7 [DOI] [PubMed] [Google Scholar]
- 83. Nguyen P, Sahara K, Yoshido A, Marec F. Evolutionary dynamics of rDNA clusters on chromosomes of moths and butterflies (Lepidoptera). Genetica. 2010;138: 343–354. 10.1007/s10709-009-9424-5 [DOI] [PubMed] [Google Scholar]
- 84. Cabral-de-Mello DC, Oliveira SG, Moura RC, Martins C. Chromosomal organization of the 18S and 5S rRNAs and histone H3 genes in Scarabaeinae coleopterans: insights into the evolutionary dynamics of multigene families and heterochromatin. BMC Genet. 2011;12: 88 10.1186/1471-2156-12-88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Panzera Y, Pita S, Ferreiro MJ, Ferrandis I, Lages C, Pérez R, et al. High dynamics of rDNA cluster location in kissing bug holocentric chromosomes (Triatominae, Heteroptera). Cytogenet Genome Res. 2012;138: 56–67. 10.1159/000341888 [DOI] [PubMed] [Google Scholar]
- 86. Utsunomia R, Scacchetti PC, Pansonato-Alves JC, Oliveira C, Foresti F. Comparative chromosome mapping of U2 snRNA and 5S rRNA genes in Gymnotus species (Gymnotiformes, Gymnotidae): evolutionary dynamics and sex chromosome linkage in G. pantanal . Cytogenet Genome Res. 2014;142: 286–292. 10.1159/000362258 [DOI] [PubMed] [Google Scholar]
- 87. Úbeda-Manzanaro M, Merlo MA, Palazón JL, Cross I, Sarasquete C, Rebordinos L. Chromosomal mapping of the major and minor ribosomal genes, (GATA)n and U2 snRNA gene by double-colour FISH in species of the Batrachoididae family. Genetica. 2010;138: 787–794. 10.1007/s10709-010-9460-1 [DOI] [PubMed] [Google Scholar]
- 88. Pelliccia F, Barzotti R, Bucciarelli E, Rocchi A. 5S ribosomal and U1 small nuclear RNA genes: a new linkage type in the genome of a crustacean that has three different tandemly repeated units containing 5S ribosomal DNA sequences. Genome. 2001;44: 331–335. [DOI] [PubMed] [Google Scholar]
- 89. Manchado M, Zuasti E, Cross I, Merlo A, Infante C, Rebordinos L. Molecular characterization and chromosomal mapping of the 5S rRNA gene in Solea senegalensis: a new linkage to the U1, U2, and U5 small nuclear RNA genes. Genome. 2006;49: 79–86. [DOI] [PubMed] [Google Scholar]
- 90. Vierna J, Jensen KT, Martínez-Lage A, González-Tizón AM. The linked units of 5S rDNA and U1 snDNA of razor shells (Mollusca: Bivalvia: Pharidae). Heredity. 2011;107: 127–142. 10.1038/hdy.2010.174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Anjos A, Ruiz-Ruano FJ, Camacho JPM, Loreto V, Cabrero J, de Souza MJ, Cabral-de-Mello DC. U1 snDNA clusters in grasshoppers: chromosomal dynamics and genomic organization. Heredity. 2015;114: 207–219. 10.1038/hdy.2014.87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Cabrero J, López-León MD, Teruel M, Camacho JP. Chromosome mapping of H3 and H4 histone gene clusters in 35 species of acridid grasshoppers. Chromosome Res. 2009;17: 397–404. 10.1007/s10577-009-9030-5 [DOI] [PubMed] [Google Scholar]
- 93. Cohen S, Agmon N, Sobol O, Segal D. Extrachromosomal circles of satellite repeats and 5S ribosomal DNA in human cells. Mobile DNA. 2010;1: 11 10.1186/1759-8753-1-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
Data Availability Statement
The data underlying the findings described in the paper is freely available to other researchers (1) in the body of the paper and (2) in the supporting information.