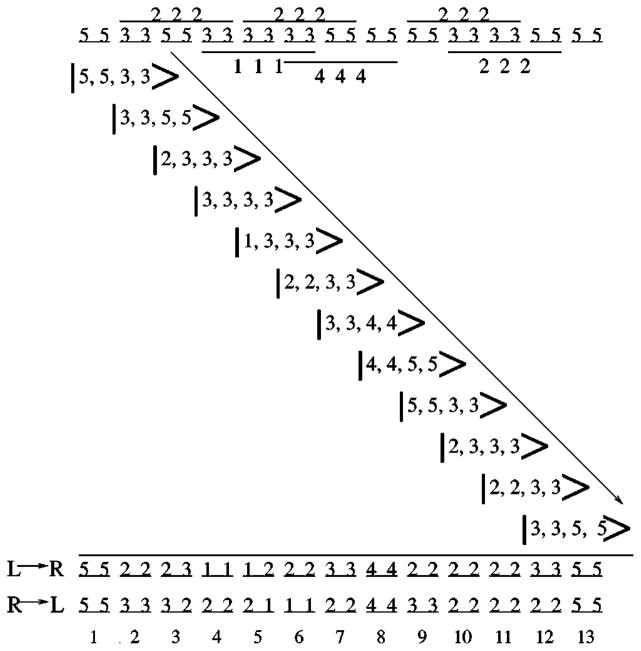
FIG. 7.
The top schematic describes the backbone of a 13-mer peptide chain in a conformation that has torsional constraints with pure entropy ranked either 3 or 5 and occasional hydrogen bond constraints (pictorially represented as bars spanning three consecutive pairs of dihedral angle dof) with pure entropy ranked either 1, 2, or 4. Each step in the propagation of the local rigidity state from left to right is shown along the diagonal. The final ranks that remain after propagating from left to right (L→R) is given on the third to last row. The final ranks obtained by propagating from right to left (R→L) are given on the second to last row. The last row labels the amino acids. Both propagation directions yield three rank-1, 12 rank-2, five rank-3, two rank-4, and four rank-5 independent distance constraints.