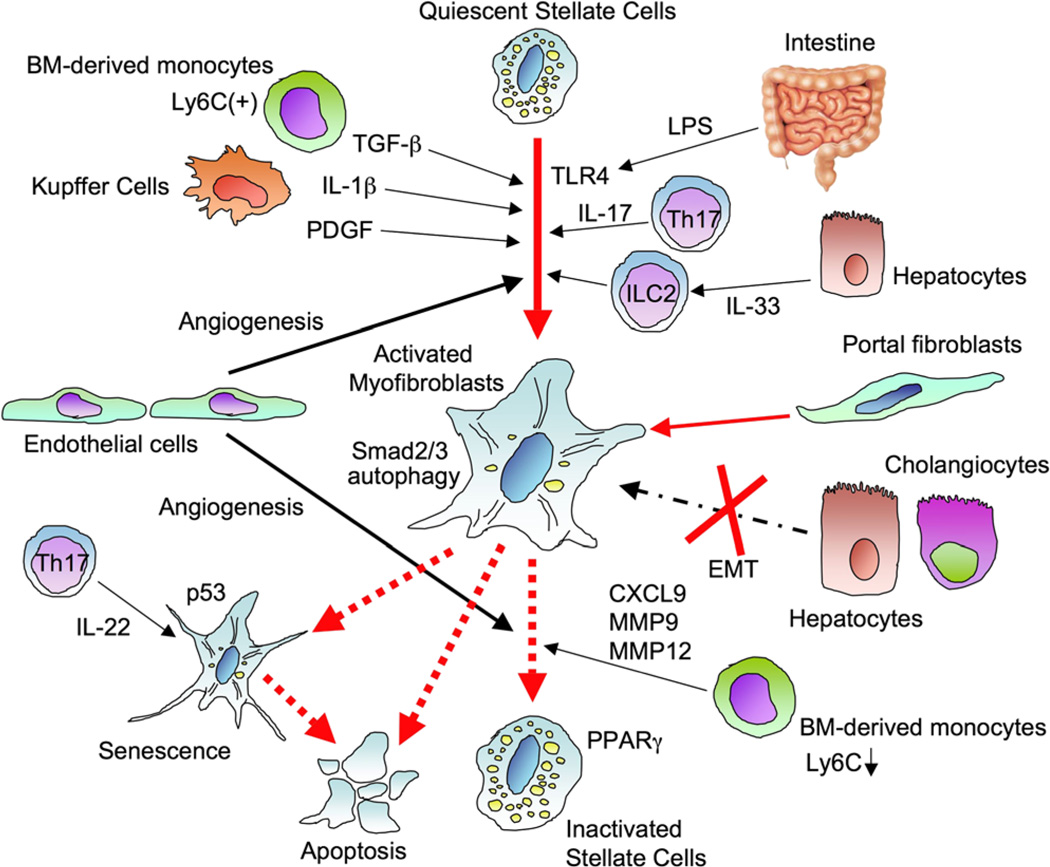
Fig. 1.
Activation and regression of hepatic stellate cells. Quiescent hepatic stellate cells (HSCs) store Vitamin A containing lipid droplets and lose Vitamin A when the cells are activated. Hepatic epithelial injury, such as death of hepatocytes and biliary epithelial cells, induces activation of HSCs directly or through cytokines released from immune cells including Kupffer cells, bone marrow-derived monocytes, Th17 cells, and innate lymphoid cells (ILC). Transforming growth factor-β (TGF-β), platelet-derived growth factor (PDGF), interleukin-1β (IL-1β), IL-17, and intestine-derived lipopolysaccharide (LPS) promote HSC activation. IL-33 promotes HSC activation through ILC2. Autophagy in HSCs is associated with HSC activation. The activated myofibroblast pool is mainly constituted by activated HSCs, but biliary injury induces differentiation of portal fibroblasts to activated myofibroblasts. However, there is no evidence of epithelial-mesenchymal transition for constituting the myofibroblast pool. After the cessation of causative liver injury, fibrosis starts regression, and activated HSCs induce apoptosis or revert into a quiescent state. Peroxisome proliferator-activated receptor γ (PPARγ) expression in HSCs is associated with HSC reversal. Some activated HSCs become senescent, resulting in loss of profibrogenic property in which p53 plays a role. Moreover, angiogenesis contributes to both fibrosis development and regression