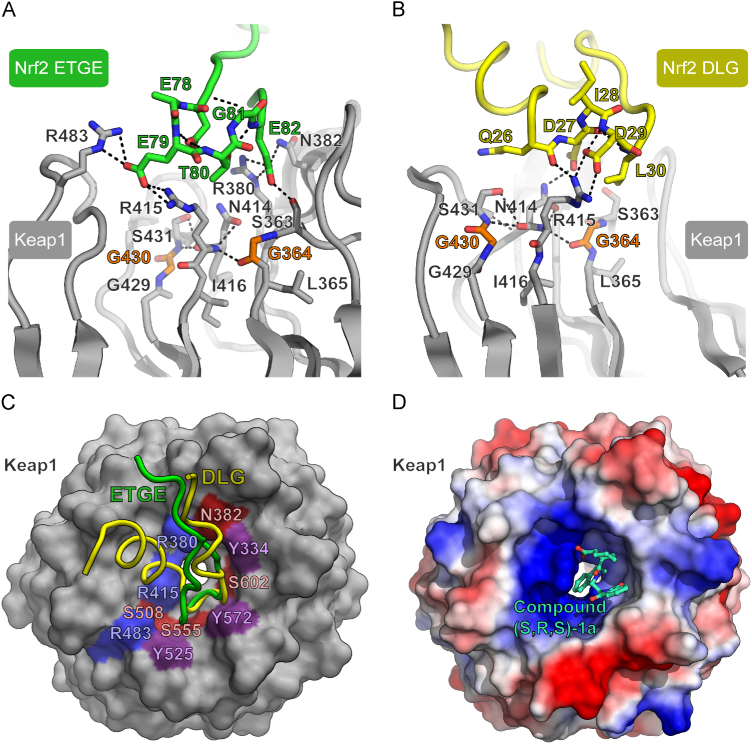
Fig. 2.
Binding of Nrf2 to Keap1. (A) Selected side-chain interactions are shown in the complex of human Keap1 and the Nrf2 ETGE motif (PDB 2FLU). Kelch domain positions with known somatic cancer mutations (G364C and G430C) are shown in orange; other Keap1 and Nrf2 interface residues are shown in gray and green, respectively. (B) Selected side-chain interactions in the DLG motif complex with mouse Keap1 (PDB 3WN7). DLG peptide residues are colored yellow; Keap1 residues are colored as in (A). (C) Comparison of the binding of the ETGE (green) and DLG (yellow) peptides. Colored areas on the Keap1 surface indicate the main interacting residues (blue, basic; red, polar; purple, hydrophobic). (D) Structural basis for Keap1 inhibition by small molecules targeting the Kelch domain. The electrostatic potential of the protein surface reveals a basic patch around the Nrf2 binding site. A bound small-molecule inhibitor is shown from PDB 4L7B (chain B) [70].