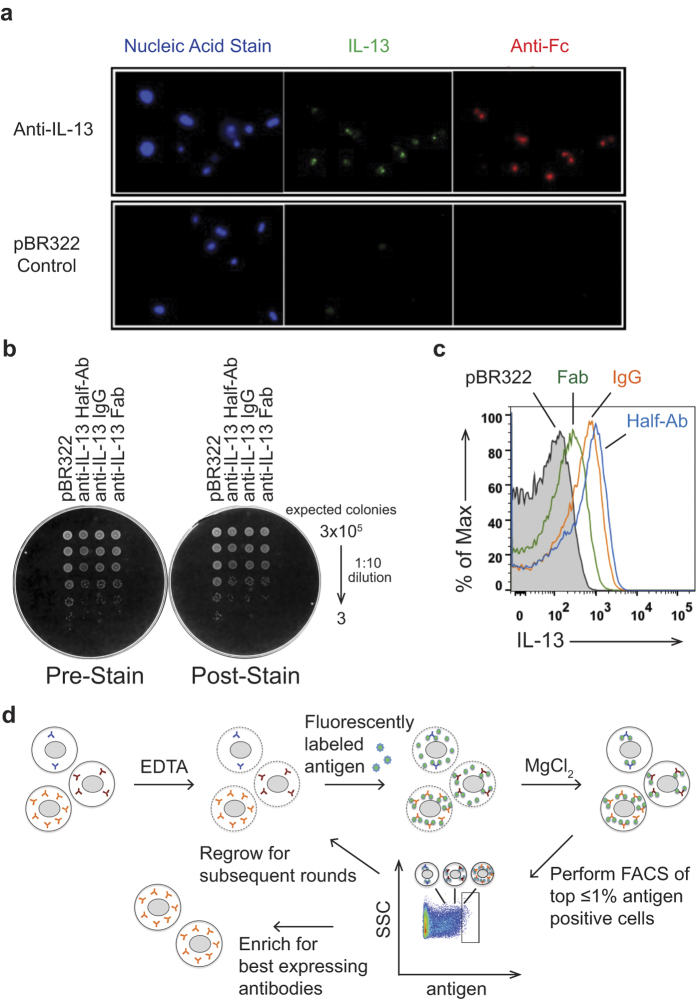
Figure 1. Antigen can be targeted to antibody expressing cells for Bacterial Antibody Display (BAD).
(A) Fluorescence microscopy visualizes specific targeting of IL-13 antigen to antibody expressing cells. Cells expressing an anti-IL-13 antibody or the empty vector control (pBR322) were stained with Syto 41 (nucleic acid stain, blue), Alexa488 labeled IL-13 (green), or DyLight649 labeled anti-human Fc F(ab’)2 (red) antibodies. Only cells expressing the antibody stain positive with IL-13 antigen and anti-Fc. (B) Viability of cells is not impacted by the staining conditions. Serial dilutions of cells expressing different anti-IL-13 antibody formats or the empty control vector (pBR322) before (Pre-Stain) and after EDTA treatment and antigen staining, followed by MgCl2 addition (Post-Stain) were plated. No difference in viability is observed between the unstained and stained cells. (C) Flow cytometric analysis of cells expressing different formats of an anti-IL-13 antibody (IgG (orange), half-antibody (blue), and Fab (green)) or pBR322 control cells (black, shaded) after staining with fluorescently labeled IL-13. All formats are suitable for BAD with a shift above the pBR322 control. (D) Schematic diagram of BAD. Cells are permeabilized by treatment with EDTA, incubated with fluorescently labeled antigen, and the integrity of the outer membrane restored by addition of MgCl2. The top ≤ 1% antigen-positive cells are sorted by flow cytometry and either progress after regrowth into a subsequent round for further enrichment or are collected for analysis.