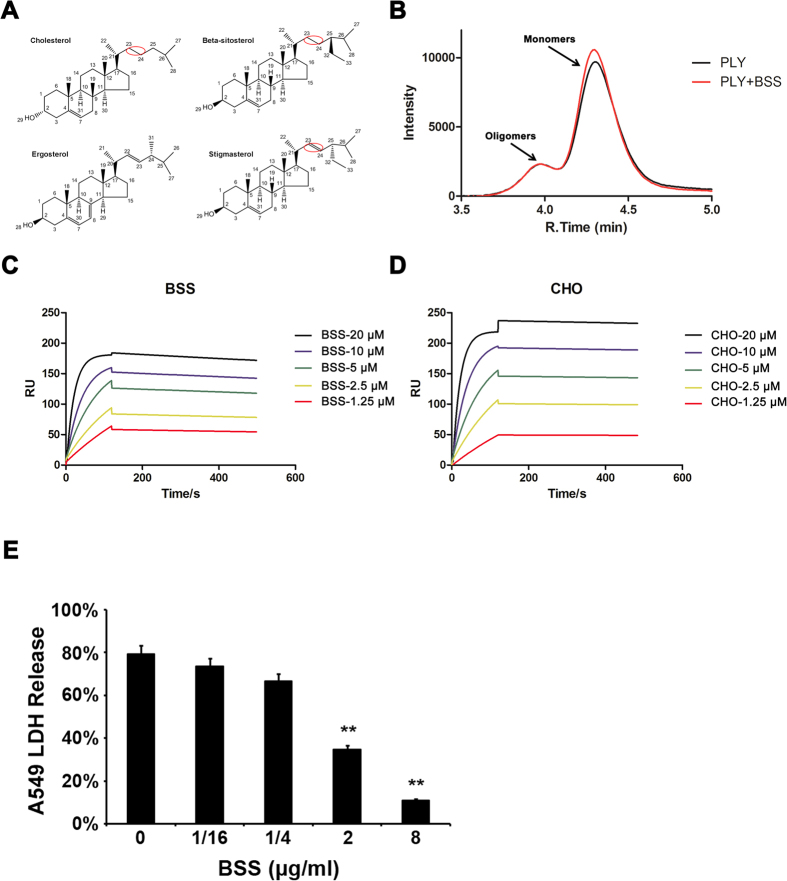
Figure 1. Chemical structures of four sterols and the inhibitory mechanism of β-sitosterol against PLY.
(A) Chemical structures of four sterols. The red circle indicates the structural differences among cholesterol, β-sitosterol and stigmasterol, which are the critical chemical bonds responsible for the binding to pneumolysin. (B) β-sitosterol did not affect the oligoerization of pneumolysin. Purified pneumolysin at a concentration capable of self-assembly (>10 mg/ml) was incubated with β-sitosterol and the mixture was analyzed by high performance liquid chromatograph (HPLC). The toxin eluted in a profile identical to the control which did not receive β-sitosterol. (C,D) The interactions of pneumolysin with cholesterol or β-sitosterol. Pneumolysin was immobilized on an SPR assay chip and liposomes containing β-sitosterol or cholesterol at the indicated concentrations were used to determine the binding. (E) β-sitosterol protects human alveolar epithelial cells from cells injury caused by PLY. A549 cells were treated with the toxin in medium supplemented with different concentrations of β-sitosterol. Cell injury was measured by the release of LDH. The values in the bars represent the means±SD of three independent experiments. *p < 0.05 and **p < 0.01.