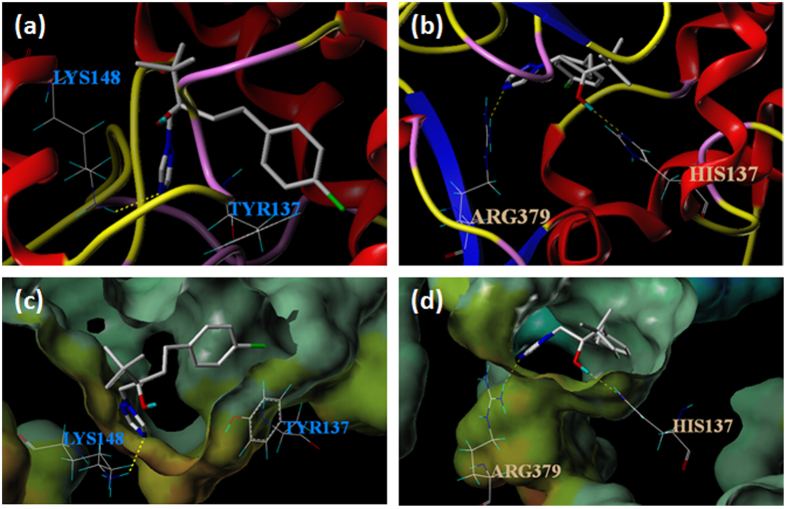
Figure 5. Molecule docking for the VvCYP51-tebuconazole complex.
(a) Tebuconazole formed hydrogen bond (dotted line) with amino acid Lys148 in VvCYP51. (b) Tebuconazole formed hydrogen bond (dotted lines) with amino acids Arg379 and His137 in VvCYP51 with Y137H. (c) The hydrophobic and electrostatic environment of binding between Lys148 and VvCYP51. (d) The hydrophobic and electrostatic environment of binding between Arg379, His137 and VvCYP51 with Y137H. The color range for hydrophobicity potential ranges from brown (highest lipophilic area of the molecule) to blue (highest hydrophilic area). Electrostatic potential ranges from red (most positive) to purple (most negative). The colors of the environment in (c,d) did not change significantly from green and beige to blue or brown, indicating that the point mutation did not significantly change the hydrophobic and electrostatic environment of binding.