Abstract
Prenatal ethanol exposure (PEE) is an established risk factor for intrauterine growth retardation. The present study was designed to determine whether PEE can increase the susceptibility of high-fat diet (HFD)-induced metabolic syndrome (MS) in adult offspring in a sex-specific manner, based on a generalized linear model analysis. Pregnant Wistar rats were administered ethanol (4 g/kg.d) from gestational day 11 until term delivery. All offspring were fed either a normal diet or a HFD after weaning and were sacrificed at postnatal week 20, and blood samples were collected. Results showed that PEE reduced serum adrenocorticotropic hormone (ACTH) and corticosterone levels but enhanced serum glucose, insulin, insulin resistant index (IRI), triglyceride and total cholesterol (TC) concentrations. Moreover, the analysis showed interactions among PEE, HFD and sex. In the PEE offspring, HFD aggravated the decrease in ACTH and corticosterone levels and further increased serum glucose, insulin, triglyceride and TC levels. The changes of serum ACTH, glucose and IRI levels in the female HFD rats were greater than those in the male HFD rats. Our findings suggest that PEE enhances the susceptibility to MS induced by HFD in a sex-specific manner, which might be primarily associated with the neuroendocrine metabolic programming by PEE.
Metabolic syndrome (MS), referring to a clustering of metabolic risk factors including central obesity, glucose intolerance, dyslipidemia and hypertension, is becoming one of the main threats to human health worldwide1. The prevalence of MS is increasing annually2. In United States, the morbidity rates associated with MS are 9.2% in adolescents3 and 22.9% in adults4. Intrauterine growth retardation (IUGR), defined as a birth weight below the 10th customized centile for gestational age, is characterized primarily by the poor growth potential of a fetus in utero5. In an epidemiological survey, the prevalence of overweight and obesity was high in adult women who were in utero during the Dutch or Chinese famine6, which also led to a higher risk of MS7. Epidemiological investigations8,9 and animal studies10,11 found that fetuses born with IUGR had a higher risk of developing MS and cardiovascular disease in adulthood, and that the incidence of MS in adults who previously experienced IUGR was 5.75-fold higher than that in healthy adults9. However, the underlying mechanisms are still unclear. To date, the most acceptable theory is the ‘thrifty phenotype hypothesis’12, which proposes that exposure of a fetus to an adverse intrauterine environment increases the sensitivity of the peripheral tissues to metabolic hormones (e.g., glucocorticoid), the increased sensitivity improves the fetal survival rate. The offspring will exhibit catch-up growth and have a higher risk of developing MS in adulthood as the nutritional pattern improves after birth13.
Prenatal ethanol exposure (PEE) is an established risk factor for several adverse birth outcomes, including multiple birth defects, mental retardation and IUGR, which are collectively known as fetal alcohol effects14,15. Many studies have shown that PEE induces IUGR16,17, which may be associated with PEE-impaired placentation16. Such IUGR offspring may present catch-up growth and develop dyslipidemia and hyperglycemia, showing increased susceptibility to MS in adulthood13,18. High-fat diet (HFD) is one of the main environmental factors accounting for the incidence of MS19. Epidemiological survey found ethanol exposure during pregnancy increases obesity rate and fat accumulation after birth20. Meanwhile, many animal studies have also found the ethanol-induced fat infiltration widely exists in pancreas, liver and subcutaneous fat21,22,23. Our previous studies confirmed that PEE increases the susceptibility to non-alcoholic fatty liver disease induced by a HFD in rat offspring23, which may be associated with fetal over-exposure to maternal glucocorticoid, resulting in both functional inhibition of the hypothalamic–pituitary–adrenal (HPA) axis24 and insulin-like growth factor 1-associated glucose and lipid metabolic alteration in peripheral tissues23. Furthermore, because these changes persist after birth, they manifest as HPA axis dysfunction and glucocorticoid-dependent blood glucose and lipid metabolic alterations in adult rats25.
Study showed that there were sex-specific changes in the key factors regulating glucose and lipid metabolism, such as insulin and adiponectin signaling pathways, which indicates different effects of the HFD on male and female rats26. Overall, PEE, HFD and sex are likely to increase the risk of MS development in IUGR offspring, however, the interactions among these three factors are unclear. In the present study, a rat IUGR model was established by PEE as previously described23, and a post-weaning HFD was used to induce MS in the offspring. Firstly, we observed alterations in HPA axis activity and glucose and lipid metabolism, including changes in blood adrenocorticotropic hormone (ACTH), corticosterone, glucose and lipid levels. Then, the interactions among PEE, HFD and sex were studied based on a generalized linear model. This study aimed to explore the risk factors for adult MS characterized by intrauterine origin and the links among them, which will be beneficial for elucidating the underlying mechanisms responsible for the susceptibility of IUGR offspring to adult MS and associated diseases. This study may also deepen our understanding of sex-specific differences in MS susceptibility of PEE rat offspring fed a HFD.
Results
Comparison between groups —Least significant difference (LSD) post hoc test Alterations in HPA axis activities
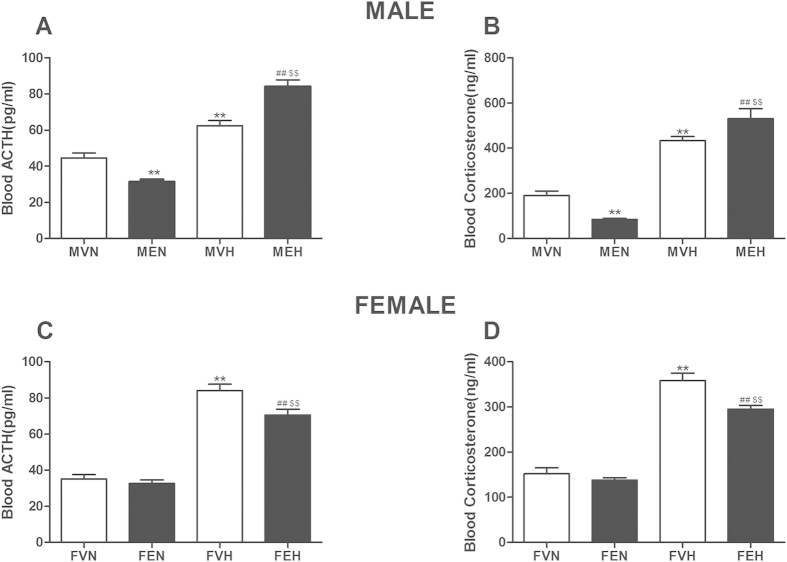
For the male offspring (as shown in Fig. 1A,B), the serum ACTH and corticosterone concentrations were lower in the male offspring by prenatal ethanol exposure with normal diet (MEN) group (P < 0.01) than in the male offspring by prenatal vehicle exposure with normal diet (MVN) group, and both concentrations were higher in the male offspring by prenatal ethanol exposure with HFD (MEH) group than in the male offspring by prenatal vehicle exposure with HFD (MVH) group (P < 0.01). In the MVH group, the serum ACTH and corticosterone levels were higher than in the MVN group (P < 0.01), and both parameters were higher in the MEH group than in the MVH group (P < 0.01).
Figure 1. Effects of prenatal ethanol (4 g/kg.d) exposure on serum adrenocorticotrophic hormone (ACTH) and corticosterone concentrations in the rat offspring.
(A) Male ACTH concentration; (B) Male corticosterone concentration; (C) Female ACTH concentration; (D) Female corticosterone concentration. Mean ± S.E.M., n = 8. **P < 0.01 MVN vs. MEN and MVH vs MEH or **P < 0.01 FVN vs. FEN and FVH vs FEH; ##P < 0.01 MVN vs. MVH and MEN vs MEH or ##P < 0.01 FVN vs. FVH and FEN vs FEH. MVN: male offspring by prenatal vehicle exposure with normal diet; MEN: male offspring by prenatal ethanol exposure with normal diet; MVH: male offspring by prenatal vehicle exposure with high-fat diet (HFD); MEH: male offspring by prenatal ethanol exposure with HFD; FVN: female offspring by prenatal vehicle exposure with normal diet; FEN: female offspring by prenatal ethanol exposure with normal diet; FVH: female offspring by prenatal vehicle exposure with HFD; FEH: female offspring by prenatal ethanol exposure with HFD.
For the female offspring (as shown in Fig. 1C,D), there were no significant differences between the female offspring by prenatal vehicle exposure with normal diet (FVN) and female offspring by prenatal ethanol exposure with normal diet (FEN) groups in terms of the serum ACTH and corticosterone levels, and the serum ACTH and corticosterone levels were lower in the female offspring by prenatal ethanol exposure with HFD (FEH) group than in the female offspring by prenatal vehicle exposure with HFD (FVH) group (P < 0.01). Meanwhile, the serum ACTH and corticosterone levels were higher in the FVH group than in the FVN group (P < 0.01), and both parameters were higher in the FEH group than in the FEN group (P < 0.01).
Alterations in serum glucose and insulin levels
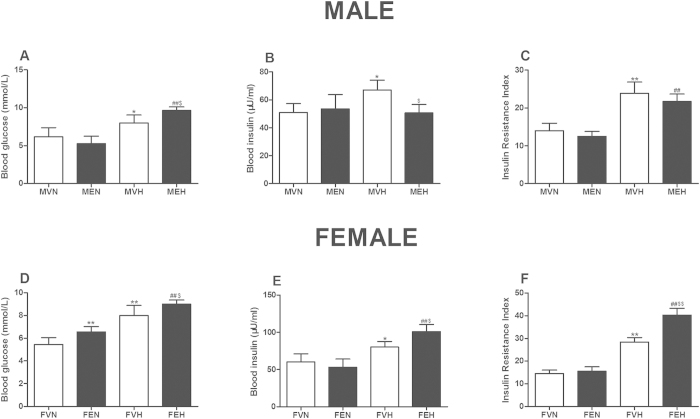
For the male offspring (as shown in Fig. 2A/B/C), there were no differences between the MVN and MEN groups in terms of the serum glucose and insulin levels or the insulin resistance index (IRI). However, the serum glucose concentration in the MEH group was markedly higher than that in the MVH group, and the serum insulin concentration in the MEH group was markedly lower than that in the MVH group (P < 0.05). Meanwhile, the serum glucose level, serum insulin concentration and IRI were higher in the MVH group than in the MVN group (P < 0.05, P < 0.01), and the serum glucose and IRI levels were higher in the MEH group than in the MEN group (P < 0.01).
Figure 2. Effects of prenatal ethanol (4 g/kg.d) exposure on serum glucose, serum insulin concentrations and insulin resistance index (IRI) in the rat offspring.
(A) Male glucose concentration; (B) Male insulin concentration; (C) Male IRI; (D) Female glucose concentration; E: Female insulin concentration; F: Female IRI. Mean ± S.E.M., n = 8. *P < 0.05, **P < 0.01 MVN vs. MEN and MVH vs MEH or *P < 0.05, **P < 0.01 FVN vs. FEN and FVH vs FEH; #P < 0.05, ##P < 0.01 MVN vs. MVH and MEN vs MEH or #P < 0.05, ##P < 0.01 FVN vs. FVH and FEN vs FEH. MVN: male offspring by prenatal vehicle exposure with normal diet; MEN: male offspring by prenatal ethanol exposure with normal diet; MVH: male offspring by prenatal vehicle exposure with high-fat diet (HFD); MEH: male offspring by prenatal ethanol exposure with HFD; FVN: female offspring by prenatal vehicle exposure with normal diet; FEN: female offspring by prenatal ethanol exposure with normal diet; FVH: female offspring by prenatal vehicle exposure with HFD; FEH: female offspring by prenatal ethanol exposure with HFD.
For the female offspring (as shown in Fig. 2D/E/F), the serum glucose level in the FEN group was higher than that in the FVN group (P < 0.01), and the serum glucose level, insulin level and IRI were higher in the FEH group than in the FVH group (P < 0.05, P < 0.01). Meanwhile, the serum glucose level, insulin level and IRI in the FVH group were higher than those in the FVN group (P < 0.05, P < 0.01), and the serum glucose level, insulin level and IRI in the FEH group were higher than those in the FEN group (P < 0.01).
Alterations in serum triglyceride and TC levels
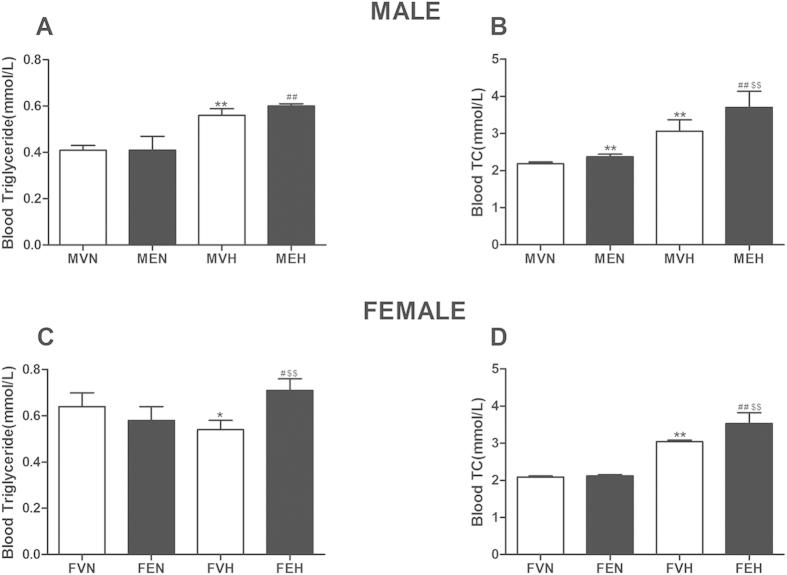
For the male offspring (as shown in Fig. 3A,B), the serum TC concentration was higher in the MEN and MEH groups than in the MVN and MVH groups, respectively (P < 0.01). Meanwhile, the serum triglyceride and TC concentrations were significantly higher in the MVH and MEH groups than in the MVN and MEN groups, respectively (P < 0.01).
Figure 3. Effects of prenatal ethanol (4 g/kg.d) exposure on serum triglyceride and cholesterol (TC) concentrations in the rat offspring.
(A) Male triglyceride concentration; (B) Male TC concentration; (C) Female triglyceride concentration; (D) Female TC concentration. Mean ± S.E.M., n = 8. **P < 0.01 MVN vs. MEN and MVH vs MEH or **P < 0.01 FVN vs. FEN and FVH vs FEH; #P < 0.05, ##P < 0.01 MVN vs. MVH and MEN vs MEH or #P < 0.05, ##P < 0.01 FVN vs. FVH and FEN vs FEH. MVN: male offspring by prenatal vehicle exposure with normal diet; MEN: male offspring by prenatal ethanol exposure with normal diet; MVH: male offspring by prenatal vehicle exposure with high-fat diet (HFD); MEH: male offspring by prenatal ethanol exposure with HFD, FVN: female offspring by prenatal vehicle exposure with normal diet; FEN: female offspring by prenatal ethanol exposure with normal diet; FVH: female offspring by prenatal vehicle exposure with HFD; FEH: female offspring by prenatal ethanol exposure with HFD.
For the female offspring (as shown in Fig. 3C,D), between the FVN and FEN groups, there were no significant differences in the serum triglyceride and TC levels, and the serum triglyceride and TC concentrations were higher in the FEH group than in the FVH group (P < 0.01). Meanwhile, the serum TC level was higher in the FVH group than in the FVN group (P < 0.01), and the serum triglyceride and TC concentrations were higher in the FEH group than in the FEN group (P < 0.05, P < 0.01).
Interactions among PEE, HFD and sex: — generalized linear model analysis Interaction in serum ACTH and corticosterone concentrations
With respect to serum ACTH level (Table 1), PEE groups were lower than in the vehicle groups (test substances P < 0.05), and HFD groups were higher than normal diet groups (Diet P < 0.01). There were effects in the PEE*HFD, HFD*sex and PEE*sex (P < 0.01), which manifested as PEE decreased serum ACTH level while HFD has the opposite effect; and serum ACTH level of female offspring was increased more obviously than that of males in the HFD groups; while in the PEE groups, the female serum ACTH level was decreased more significantly than the male. Moreover, there was an interaction in PEE*HFD*sex (P < 0.01).
Table 1. Interactional analysis with generalized linear model among ethanol, high-fat diet (HFD) and sex on neuroendocrine metabolism-related blood phenotypes in rat offspring.
| Indexs | Factors |
|||||||
|---|---|---|---|---|---|---|---|---|
| Sex | Ethanol | HFD | Ethanol *HFD | Ethanol *Sex | HFD* Sex | Ethanol *HFD*Sex | ||
| ACTH | B | 9.49 | −2.51 | 48.98 | −11.12 | −10.72 | −31.25 | 46.13 |
| Sig | P > 0.05 | P < 0.05 | P < 0.01 | P < 0.01 | P < 0.01 | P < 0.01 | P < 0.01 | |
| Corticosterone | B | 37.81 | −14.75 | 205.4 | −4.21 | −90.90 | 37.70 | 189.21 |
| Sig | P < 0.05 | P > 0.05 | P < 0.01 | P < 0.01 | P > 0.05 | P < 0.01 | P < 0.05 | |
| Glucose | B | 0.71 | 1.10 | 2.53 | −0.09 | −2.02 | −0.65 | 2.33 |
| Sig | P > 0.05 | P < 0.01 | P < 0.01 | P < 0.01 | P < 0.05 | P > 0.05 | P < 0.01 | |
| Insulin | B | 1.43 | −3.10 | 42.23 | 10.05 | −4.23 | −0.65 | 12.50 |
| Sig | P > 0.05 | P < 0.05 | P < 0.01 | P < 0.01 | P > 0.05 | P > 0.05 | P < 0.05 | |
| Triglyceride | B | −0.23 | −0.06 | −0.07 | 0.20 | 0.05 | 0.21 | −0.15 |
| Sig | P < 0.05 | P < 0.05 | P < 0.01 | P < 0.05 | P > 0.05 | P < 0.01 | P < 0.01 | |
| TC | B | 0.09 | 0.02 | 0.95 | 0.47 | 0.17 | 0.08 | 0.03 |
| Sig | P < 0.1 | P < 0.01 | P < 0.01 | P < 0.01 | P > 0.05 | P > 0.05 | P > 0.05 | |
ACTH: adrenocorticotropic hormone; B: regression coefficient. Sig: P value, TC: total cholesterol.
With respect to serum corticosterone levels (Table 1), when compared to normal diet, it was increased in HFD groups (Diet P < 0.01), and the female groups were lower than male groups (Sex P < 0.01). There were interactions in PEE*HFD and HFD*sex (P < 0.01), which indicated that PEE*HFD and HFD*sex decreased serum corticosterone level. Moreover, there was an interaction in PEE*HFD*sex (P < 0.01).
Interaction in serum glucose and insulin concentrations
The results of glucose levels (Table 1) showed that, PEE groups were higher than vehicle groups (P < 0.01), and HFD groups were higher than normal diet ones (P < 0.01). There were interactions in PEE*HFD and PEE*sex (P < 0.05, P < 0.01), where PEE*HFD decreased serum glucose level; and this decrease was more obvious in female offspring than in the males. Moreover, there was an interaction in PEE*HFD*sex (P < 0.01).
With respect to serum insulin level (Table 1), PEE groups were lower than vehicle groups (Test substances P < 0.05), and HFD groups were higher than normal diet groups (Diet P < 0.01). There were interactions in PEE*HFD (P < 0.01), where PEE*HFD increased serum insulin level. Moreover, there was an interaction in PEE*HFD*sex (P < 0.05), the serum insulin level was increased more obviously in the female than that of the male in the PEE offspring with HFD.
Interaction in serum triglyceride and TC concentration
For the serum triglyceride level (Table 1): PEE groups were significantly higher than vehicle groups (P < 0.05), HFD groups were also higher than normal diet groups (P < 0.01). Female groups were higher than male groups (P < 0.01). There were interactions in PEE*HFD and HFD*sex (P < 0.05, P < 0.01), where PEE*HFD and HFD*sex both aggravated the increase in serum triglyceride level. Moreover, there was an interaction in PEE*HFD*sex (P < 0.01).
For the serum TC level (Table 1): PEE groups were higher than vehicle groups (P < 0.01), while compared with normal diet, HFD groups were significantly lower (P < 0.01), and female groups had an decreasing trend than male groups (P = 0.06). There was an interaction in PEE*HFD (P < 0.01), reflecting by PEE*HFD aggravated the increase of serum TC level.
Discussion
It is known that PEE, HFD and sex play important roles in the fetal origin of MS23,27,28. PEE can enhance the susceptibility to MS in female adult offspring23, and a HFD can lead to obesity, insulin resistance (IR) and other metabolic diseases29,30. Early metabolic programming can contribute to the variability observed in response to nutritional interventions and can further the development of metabolic diseases in later life, particularly in environments in which a HFD is prevalent31. In this study, we found that PEE increased the serum glucose and TC levels, and a HFD increased the levels of all blood phenotypes except for triglycerides. Sex-specific differences were observed in all blood phenotypes, with the exception of serum ACTH, insulin and glucose levels. Therefore, we speculated that PEE, HFD and sex are important factors in the developmental origins of fetal-originated MS.
In the present study, based on generalized linear model analysis, we observed the interaction of the combination of PEE and HFD-decreased serum ACTH, corticosterone and glucose levels, with enhanced serum insulin, triglyceride and TC levels. Additionally, compared with the MVH and FVH groups, the serum ACTH, corticosterone, glucose, triglycerides and TC levels were significantly increased in the MEH and FEH groups, respectively. These results suggest that combined with a HFD, the effects of PEE on serum ACTH and corticosterone may be related to the basal activity of the HPA axis, which may be increased by a HFD to promote the synthesis and secretion of hypothalamic corticotrophin-releasing hormone (CRH) in PEE offspring32. Based on the HPA axis-associated neuroendocrine metabolic programming mechanism through PEE33, we expected PEE to reduce serum triglyceride and TC levels with the increased activity of the HPA axis; however, the levels of serum triglyceride and TC were enhanced in the present study, probably due to the HFD per se (which contained 11.5% lard and 0.5% cholesterol)34. Studies have shown that a HFD increases serum insulin levels35, and PEE decreases these levels by damaging pancreatic β cells36. Here we found that PEE decreased serum insulin levels and a HFD increased these levels, which is consistent with the above-mentioned studies (Table 1), however, the interaction between PEE and HFD increased the blood insulin concentration, which suggested that the PEE offspring fed by HFD may develop insulin resistance.
Studies have identified sex disparities in the amygdalar neuronal plasticity of adult rats exposed to ethanol37. The interaction between sex and PEE was also investigated in the present study. We found that in the females, the serum glucose level was increased in the FEN group compared with the FVN group, whereas in the males, these levels were decreased in the MEN group compared with the MVN for the PEE offspring fed normal diets. Furthermore, there was an interaction between PEE and sex (not considering the HFD) based on the generalized linear model analysis, which primarily suggests that the levels of serum ACTH and glucose increased to a greater degree in the female PEE offspring. These results indicated that the combination of PEE and sex changed the HPA axis-associated neuroendocrine metabolic programming. The different sensitivities of the HPA axis in the female and male IUGR offspring rats may be involved in the observed sex differences38. The corticosterone feedback regulation (by binding to the glucocorticoid receptor) is an important mode of HPA axis regulation39, and there are sex differences in corticosterone actions40. Therefore, in terms of sex differences, corticosterone may play a role in the HPA axis. In addition, the corticosterone-related sex difference was probably caused by the regulation of sex hormones41 and the sex dependent expression of corticosterone metabolic enzymes42. Studies have shown that androgen and estrogen can affect glucose/lipid metabolism in peripheral organs (e.g., the liver) through differential regulation of the HPA axis43,44. Therefore, this sex disparity in metabolic programming by PEE was probably related to the effects of sex hormones on glucose and lipid metabolic functions.
According to other reports, sex disparities in emotional profiles and metabolic functionality have been found in the prenatal HFD-exposed mice offspring45, and a HFD can increase ovulatory dysfunction in postpubertal female rats, ultimately resulting in adverse metabolic and reproductive outcomes in female offspring46. In addition, HPA axis-associated neuroendocrine dysfunction induced by HFD is more severe in female rats when the glucocorticoid receptor expression levels are decreased in the hypothalamus, which suggests that the female is more sensitive to fat-induced nutritional imbalance47. In the present study, we found that, compared with the FVH group, serum triglyceride levels in the females demonstrated an increasing trend in the FVN group, whereas in the males, these levels were decreased in the MVN group comparing with the MVH group. Furthermore, there was an interaction between HFD and sex, which decreased the serum ACTH levels in the male offspring rats and increased the serum corticosterone and triglyceride levels in the female offspring rats. In the HFD groups, the decreased serum ACTH level and the increased serum corticosterone and triglyceride levels in the females were much more remarkable than those in the males. These results suggested that HFD-induced alteration of the HPA axis function was characterized by significant sex differences.
Few studies have explored the interactions between PEE, HFD and sex. In the present study, based on the generalized linear model analysis, the interaction between PEE and HFD increased the serum ACTH, corticosterone, insulin, and glucose levels in the female offspring and decreased the serum triglyceride levels in the male offspring, which suggests that PEE enhanced the susceptibility to MS in the adult female offspring fed a post-weaning HFD. In addition, we found that serum glucose levels increased but corticosterone levels decreased in the PEE female offspring fed a HFD. In terms of how reduced serum cortisol/ACTH levels increased the serum glucose levels in the female offspring in this study, we found that the serum corticosterone and glucose levels were higher in the HFD group than in the rats fed a normal diet, without sex differences observed, which is consistent with other reports48,49. Meanwhile, it has been shown that fetal pancreas development is damaged in the PEE offspring rats, which can lead to increased serum glucose levels at 6 months of age50. In the present study, we observed decreased serum corticosterone levels and increased glucose levels in the rats fed HFDs, primarily in the PEE female offspring compared with the prenatal vehicle exposure female offspring. Therefore, this result may be caused by the combined effect of PEE and a HFD.
Conclusions
In this study, we demonstrated that PEE enhanced the susceptibility to MS induced by HFD in a sex-specific manner, which was probably associated with neuroendocrine metabolic programming by PEE. Furthermore, several significant interactions existed among PEE, HFD and sex, which presented as HFD aggravated PEE-associated neuroendocrine metabolic programming with greater changes observed in females. This study may provide an experimental basis for confirming the impact factors (such as PEE, HFD and sex) and their interactions in adult MS, while expanding our understanding of the negative effects of nutritional intervention on PEE offspring. Increased knowledge of these subjects may be useful for preventing and/or retarding the development of adult metabolic diseases.
Materials and Methods
Chemicals and reagents
Ethanol (analytical purity) was obtained from Zhen Xin Co., Ltd. (Shanghai, China). Isoflurane was purchased from Baxter Healthcare Co. (Deerfield, IL, USA). The rat ACTH and insulin radioimmunoassay kits were obtained from Beijing North Biotech Institute (Beijing, China). The ELISA kit for the determination of the serum corticosterone concentration was purchased from Assaypro (St Charles, USA). The glucose oxidase assay kit was provided by Shanghai Mind Bioengineering Co., Ltd. (Shanghai, China). The triglyceride and total cholesterol (TC) assay kits were obtained from Sangon Biotech Co., Ltd. (Shanghai, China). All other chemicals and reagents were of analytical grade.
Animals and treatment
The animal care protocol was approved by the Committee on the Ethics of Animal Experiments of the Wuhan University School of Medicine (Permit Number: 14016). Specific pathogen free (SPF) Wistar rats (females weighing 200–240 g; males weighing 260–300 g) were obtained from the Experimental Center of Hubei Medical Scientific Academy (No. 2008–0005, Hubei, China). Animal experiments were performed at the Center for Animal Experiment of Wuhan University (Wuhan, China), which is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC International). All animal experimental procedures were performed in accordance with the Guidelines for the Care and Use of Laboratory Animals of the Chinese Animal Welfare Committee and the International Council on Research Animal Care.
Animals were kept under temperature-controlled conditions on a 12 hour light: dark cycle with ad libitum access to standard chow and tap water at all time. After one week of acclimation, two females were mated with one male for one night. Upon confirmation of mating based on the appearance of sperm in a vaginal smear, the day was designated GD 0. Pregnant females were then transferred to individual cages.
Pregnant rats were randomly divided into two groups: control and PEE groups, and 8–10 dams were in each group. Starting from GD11 until term delivery (GD21), the PEE group was administered with oral gavage at a dose of 4 g/kg.d (36%, 14 ml/kg) ethanol, as previously described51. The control group was given the same volume of distilled water. Feed intake and weight gain of PEE-treated group during pregnancy were similar to control.
At parturition, the dams and their offspring were fed ad libitum. On postnatal day 1 (PD 1), the numbers of pups in each litter were selected to 8 pups randomly, to ensure adequate and standardized nutrition until weaning47. At PW 4, 32 pups from 8 different mothers were selected randomly for each group (16 male and 16 female IUGR pups from the ethanol group, 16 male and 16 female normal pups from the control group), and all pups were fed either a normal diet or a HFD ad libitum before being sacrificed. The standard rodent chow purchased from the Experimental Centre of Hubei Medical Scientific Academy contained 21% kcal from protein, 68.5% kcal from carbohydrate and 10.5% kcal from fat. The HFD was previously reported by our laboratory49 and contained 88.0% corn flour, 11.5% lard, and 0.5% cholesterol, which provided 18.9% kcal from protein, 61.7% kcal from carbohydrate and 19.4% kcal from fat.
At PW 20, all the rat offspring were fasted overnight after 8 p.m., then anesthetized with isoflurane and decapitated the next morning between 8 a.m. and 10 a.m. in a room separate from the room in which the other animals were kept. Trunk blood was collected and serum was prepared by centrifugation at 17,205 × g for 15 min at 4 °C. Serum samples were stored at −80 °C until they were used for the measurement of ACTH, corticosterone, glucose, insulin, triglyceride and TC concentrations.
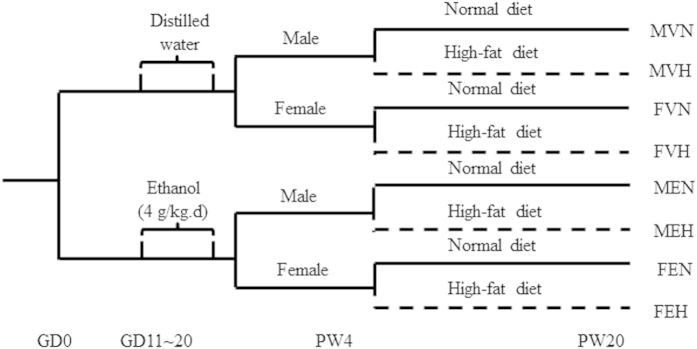
Study Design
A schematic representation of the procedure for maternal and offspring rat treatment was shown in Fig. 4. Naming principles are as follows: male offspring by prenatal vehicle exposure with normal diet (MVN), male offspring by prenatal ethanol exposure with normal diet (MEN), male offspring by prenatal vehicle exposure with HFD (MVH), male offspring by prenatal ethanol exposure with HFD (MEH), female offspring by prenatal vehicle exposure with normal diet (FVN), female offspring by prenatal ethanol exposure with normal diet (FEN), female offspring by prenatal vehicle exposure with HFD (FVH), female offspring by prenatal ethanol exposure with HFD (FEH).
Figure 4. The schedule of animal treatment from gestation day 0 (GD 0) to postnatal week 20 (PW 20).
MVN: male offspring by prenatal vehicle exposure with normal diet; MEN: male offspring by prenatal ethanol exposure with normal diet; MVH: male offspring by prenatal vehicle exposure with high-fat diet (HFD); MEH: male offspring by prenatal ethanol exposure with HFD; FVN: female offspring by prenatal vehicle exposure with normal diet; FEN: female offspring by prenatal ethanol exposure with normal diet; FVH: female offspring by prenatal vehicle exposure with HFD; FEH: female offspring by prenatal ethanol exposure with HFD.
Analysis of blood samples
The concentrations of serum ACTH and corticosterone were measured via an isotope labeling kit and an ELISA kit, respectively, following the manufacturers’ protocols. The intra-assay coefficients of variation for ACTH and corticosterone determination were 10% and 15%, respectively, and the inter-assay coefficients of variation were 5.0% and 7.2%, respectively. The concentrations of serum glucose, triglyceride and TC were measured using the respective biochemical assay kits following the manufacturers’ protocols. Serum insulin was determined using a radioimmunoassay kit. The IRI were calculated as described below.
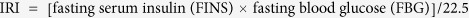 |
Statistical analysis
SPSS 18 (SPSS Science Inc., Chicago, IL, USA) and Prism (GraphPad Software, La Jolla, CA, USA) were used for data analysis. All data are presented as the mean ± S.E.M. To ensure the results were described comprehensively, we used LSD post hoc test for statistical significance among multi-groups comparisons as others used52 to analyze the effect of ethanol exposure and diet in female and male rats respectively. For example, the effect of ethanol exposure was analyzed between MVN and MEN, and the effect of diet was analyzed between MVN and MVH.
Generalized linear model analysis was used to explore the interactions among PEE, diet and sex in the development of MS, including the main effects and interaction effects of two or three factors. Sex was coded as 1for female offspring and 0 for male offspring. The interaction means the combination effects of PEE and HFD, HFD and sex, PEE and sex, as well as PEE, HFD and sex, which were abbreviated as PEE*HFD, HFD*sex PEE*sex and PEE*HFD*sex, respectively. Meanwhile, we presented a table (Table 1) with the results of P values (representing statistical significance) and B values (i.e., the regression coefficient, which reflect the effects of interaction on the dependent variable) for the analyses. If the B value is positive, it suggests that the influence factors increase the change in the dependent variable; vice versa, the influence factors decrease the change of the dependent variable when the B value is negative.
Additional Information
How to cite this article: He, Z. et al. Sex-specific increase in susceptibility to metabolic syndrome in adult offspring after prenatal ethanol exposure with post-weaning high-fat diet. Sci. Rep. 5, 17679; doi: 10.1038/srep17679 (2015).
Acknowledgments
This work was supported by grants from the National Science & Technology Pillar Program of China (No. 2013BAI12B01-3), and National Natural Science Foundation of China (Nos 81220108026, 81430089, 30830112, 81072709).
Footnotes
Author Contributions Z.H., J.L. and H.L. contributed to the acquisition of animal data. Z.H. and J.L. performed the experimental design, interpretation and manuscript writing. L.Z. and L.M. assisted with data analysis and statistical methods. L.C. and H.W. provided critical revision of the manuscript for important intellectual content. Z.H., J.L. and H.W. participated in the experimental design, the supervision, interpretation and manuscript writing.
References
- Eckel R. H., Grundy S. M. & Zimmet P. Z. The metabolic syndrome. Lancet. 365, 1415–28 (2005). [DOI] [PubMed] [Google Scholar]
- Ford E. S., Li C., Cook S. & Choi H. K. Serum concentrations of uric acid and the metabolic syndrome among US children and adolescents. Circulation. 115, 2526–32 (2007). [DOI] [PubMed] [Google Scholar]
- Girvalaki C. et al. Trends in metabolic syndrome risk factors among adolescents in rural Crete between 1989 and 2011. Hormones (Athens). 13, 259–67 (2014). [DOI] [PubMed] [Google Scholar]
- Beltran-Sanchez H., Harhay M. O., Harhay M. M. & McElligott S. Prevalence and trends of metabolic syndrome in the adult U.S. population, 1999-2010. J Am Coll Cardiol. 62, 697–703 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez M. et al. Risk of intrauterine growth restriction among HIV-infected pregnant women: a cohort study. Eur J Clin Microbiol Infect Dis. 34, 223–30 (2014). [DOI] [PubMed] [Google Scholar]
- Ravelli A. C., van Der Meulen J. H., Osmond C., Barker D. J. & Bleker O. P. Obesity at the age of 50 y in men and women exposed to famine prenatally. Am J Clin Nutr. 70, 811–6 (1999). [DOI] [PubMed] [Google Scholar]
- Zheng X. et al. Risk of metabolic syndrome in adults exposed to the great Chinese famine during the fetal life and early childhood. Eur J Clin Nutr. 66, 231–6 (2012). [DOI] [PubMed] [Google Scholar]
- Hernandez M. I. & Mericq V. Metabolic syndrome in children born small-for-gestational age. Arq Bras Endocrinol Metabol. 55, 583–9 (2011). [DOI] [PubMed] [Google Scholar]
- Alisi A., Panera N., Agostoni C. & Nobili V. Intrauterine growth retardation and nonalcoholic Fatty liver disease in children. Int J Endocrinol. 2011, 269853, doi: 10.1155/2011/269853 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohi G., Marchand K., Revesz A., Arany E. & Hardy D. B. Maternal protein restriction elevates cholesterol in adult rat offspring due to repressive changes in histone modifications at the cholesterol 7alpha-hydroxylase promoter. Mol Endocrinol. 25, 785–98 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panchal S. K. & Brown L. Rodent models for metabolic syndrome research. J Biomed Biotechnol. 2011, 351982, doi: 10.1155/2011/351982 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaquet D., Gaboriau A., Czernichow P. & Levy-Marchal C. Insulin resistance early in adulthood in subjects born with intrauterine growth retardation. J Clin Endocrinol Metab. 85, 1401–6 (2000). [DOI] [PubMed] [Google Scholar]
- Morrison J. L., Duffield J. A., Muhlhausler B. S., Gentili S. & McMillen I. C. Fetal growth restriction, catch-up growth and the early origins of insulin resistance and visceral obesity. Pediatr Nephrol. 25, 669–77 (2010). [DOI] [PubMed] [Google Scholar]
- Mullally A., Cleary B. J., Barry J., Fahey T. P. & Murphy D. J. Prevalence, predictors and perinatal outcomes of peri-conceptional alcohol exposure–retrospective cohort study in an urban obstetric population in Ireland. BMC Pregnancy Childbirth. 11, 27, doi: 10.1186/1471-2393-11-27 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy D. J., Mullally A., Cleary B. J., Fahey T. & Barry J. Behavioural change in relation to alcohol exposure in early pregnancy and impact on perinatal outcomes–a prospective cohort study. BMC Pregnancy Childbirth. 13, 8, doi: 10.1186/1471-2393-13-8 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundogan F. et al. Impaired placentation in fetal alcohol syndrome. Placenta. 29, 148–57 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W., Gregg J. P. & Fonkalsrud E. W. Effect of maternal ethanol intake on fetal rabbit gastrointestinal development. J Pediatr Surg. 29, 1030–3, discussion 1033-4 (1994). [DOI] [PubMed] [Google Scholar]
- Tosh D. N. et al. Epigenetics of programmed obesity: alteration in IUGR rat hepatic IGF1 mRNA expression and histone structure in rapid vs. delayed postnatal catch-up growth. Am J Physiol Gastrointest Liver Physiol. 299, G1023–9 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touati S. et al. Exercise reverses metabolic syndrome in high-fat diet-induced obese rats. Med Sci Sports Exerc. 43, 398–407 (2011). [DOI] [PubMed] [Google Scholar]
- Werts R. L., Van Calcar S. C., Wargowski D. S. & Smith S. M. Inappropriate feeding behaviors and dietary intakes in children with fetal alcohol spectrum disorder or probable prenatal alcohol exposure. Alcohol Clin Exp Res. 38, 871–8 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson C. C. et al. Chronic prenatal ethanol exposure increases adiposity and disrupts pancreatic morphology in adult guinea pig offspring. Nutr Diabetes. 2, e57 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdge G. C. & Postle A. D. Effect of maternal ethanol consumption during pregnancy on the phospholipid molecular species composition of fetal guinea-pig brain, liver and plasma. Biochim Biophys Acta. 1256, 346–52 (1995). [DOI] [PubMed] [Google Scholar]
- Shen L. et al. Prenatal ethanol exposure programs an increased susceptibility of non-alcoholic fatty liver disease in female adult offspring rats. Toxicol Appl Pharmacol. 274, 263–73 (2014). [DOI] [PubMed] [Google Scholar]
- Liang G., Chen M., Pan X. L., Zheng J. & Wang H. Ethanol-induced inhibition of fetal hypothalamic-pituitary-adrenal axis due to prenatal overexposure to maternal glucocorticoid in mice. Exp Toxicol Pathol. 63, 607–11 (2011). [DOI] [PubMed] [Google Scholar]
- Liu Y. et al. Fetal rat metabonome alteration by prenatal caffeine ingestion probably due to the increased circulatory glucocorticoid level and altered peripheral glucose and lipid metabolic pathways. Toxicol Appl Pharmacol. 262, 205–16 (2012). [DOI] [PubMed] [Google Scholar]
- Amengual-Cladera E., Llado I., Gianotti M. & Proenza A. M. Sex differences in the effect of high-fat diet feeding on rat white adipose tissue mitochondrial function and insulin sensitivity. Metabolism. 61, 1108–17 (2012). [DOI] [PubMed] [Google Scholar]
- Fan J. G. & Cao H. X. Role of diet and nutritional management in non-alcoholic fatty liver disease. J Gastroenterol Hepatol. 28 Suppl 4, 81–7 (2013). [DOI] [PubMed] [Google Scholar]
- Liu J. et al. Intrauterine growth retardation increases the susceptibility of pigs to high-fat diet-induced mitochondrial dysfunction in skeletal muscle. PLoS. One. 7, e34835 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinonen I. et al. The effects of equal caloric high fat and western diet on metabolic syndrome, oxidative stress and vascular endothelial function in mice. Acta Physiol (Oxf). 211, 515–27 (2014). [DOI] [PubMed] [Google Scholar]
- Panchal S. K. et al. High-carbohydrate, high-fat diet-induced metabolic syndrome and cardiovascular remodeling in rats. J Cardiovasc Pharmacol. 57, 611–24 (2011). [DOI] [PubMed] [Google Scholar]
- Rueda-Clausen C. F. et al. Hypoxia-induced intrauterine growth restriction increases the susceptibility of rats to high-fat diet-induced metabolic syndrome. Diabetes. 60, 507–16 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin A. C. et al. Chronic exposure to a high-fat diet affects stress axis function differentially in diet-induced obese and diet-resistant rats. Int J Obes (Lond). 34, 1218–26 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D. et al. A hypothalamic-pituitary-adrenal axis-associated neuroendocrine metabolic programmed alteration in offspring rats of IUGR induced by prenatal caffeine ingestion. Toxicol Appl Pharmacol. 264, 395–403 (2012). [DOI] [PubMed] [Google Scholar]
- Zhang L. et al. Prenatal food restriction induces a hypothalamic-pituitary-adrenocortical axis-associated neuroendocrine metabolic programmed alteration in adult offspring rats. Arch Med Res. 44, 335–45 (2013). [DOI] [PubMed] [Google Scholar]
- Liu Z. et al. High-fat diet induces hepatic insulin resistance and impairment of synaptic plasticity. PLoS One. 10, e0128274 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S. et al. Ethanol induced impairment of glucose metabolism involves alterations of GABAergic signaling in pancreatic beta-cells. Toxicology. 326, 44–52 (2014). [DOI] [PubMed] [Google Scholar]
- Retson T. A., Hoek J. B., Sterling R. C. & Van Bockstaele E. J. Amygdalar neuronal plasticity and the interactions of alcohol, sex, and stress. Brain Struct Funct. 4, 851, doi: 10.1007/s00429-014-0851-4 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingas R. I. & Matthews S. G. A short period of maternal nutrient restriction in late gestation modifies pituitary-adrenal function in adult guinea pig offspring. Neuroendocrinology. 73, 302–11 (2001). [DOI] [PubMed] [Google Scholar]
- van der Knaap L. J., Oldehinkel A. J., Verhulst F. C., van Oort F. V. & Riese H. Glucocorticoid receptor gene methylation and HPA-axis regulation in adolescents. The TRAILS study. Psychoneuroendocrinology. 58, 46–50 (2015). [DOI] [PubMed] [Google Scholar]
- Quinn M., Ramamoorthy S. & Cidlowski J. A. Sexually dimorphic actions of glucocorticoids: beyond chromosomes and sex hormones. Ann N Y Acad Sci. 1317, 1–6 (2014). [DOI] [PubMed] [Google Scholar]
- van Lier E., Carriquiry M. & Meikle A. Sex steroid modulation of cortisol secretion in sheep. Animal. 8, 960–7 (2014). [DOI] [PubMed] [Google Scholar]
- Hassan-Smith Z. K. et al. Gender-Specific Differences in Skeletal Muscle 11beta-HSD1 Expression Across Healthy Aging. J Clin Endocrinol Metab. 100, 2673–81 (2015). [DOI] [PubMed] [Google Scholar]
- Weiser M. J. & Handa R. J. Estrogen impairs glucocorticoid dependent negative feedback on the hypothalamic-pituitary-adrenal axis via estrogen receptor alpha within the hypothalamus. Neuroscience. 159, 883–95 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viau V. & Meaney M. J. Testosterone-dependent variations in plasma and intrapituitary corticosteroid binding globulin and stress hypothalamic-pituitary-adrenal activity in the male rat. J Endocrinol. 181, 223–31 (2004). [DOI] [PubMed] [Google Scholar]
- Bellisario V. et al. Gender-dependent resiliency to stressful and metabolic challenges following prenatal exposure to high-fat diet in the p66(Shc-/-) mouse. Front Behav Neurosci. 8, 285, doi: 10.3389/fnbeh.2014.00285 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds C. M., Segovia S. A., Zhang X. D., Gray C. & Vickers M. H. Conjugated Linoleic Acid Supplementation During Pregnancy and Lactation Reduces Maternal High-Fat Diet Induced Programming of Early Onset Puberty and Hyperlipidemia in Female Rat Offspring. Biol Reprod. 92, 40, doi: 10.1095/biolreprod.114.125047 (2014). [DOI] [PubMed] [Google Scholar]
- Boukouvalas G., Antoniou K., Papalexi E. & Kitraki E. Post weaning high fat feeding affects rats’ behavior and hypothalamic pituitary adrenal axis at the onset of puberty in a sexually dimorphic manner. Neuroscience. 153, 373–82 (2008). [DOI] [PubMed] [Google Scholar]
- Ikemoto S. et al. High-fat diet-induced hyperglycemia and obesity in mice: differential effects of dietary oils. Metabolism. 45, 1539–46 (1996). [DOI] [PubMed] [Google Scholar]
- Winzell M. S. & Ahren B. The high-fat diet-fed mouse: a model for studying mechanisms and treatment of impaired glucose tolerance and type 2 diabetes. Diabetes. 53 Suppl 3, S215–9 (2004). [DOI] [PubMed] [Google Scholar]
- Chen L. & Nyomba B. L. Effects of prenatal alcohol exposure on glucose tolerance in the rat offspring. Metabolism. 52, 454–62 (2003). [DOI] [PubMed] [Google Scholar]
- Dembele K., Yao X. H., Chen L. & Nyomba B. L. Intrauterine ethanol exposure results in hypothalamic oxidative stress and neuroendocrine alterations in adult rat offspring. Am J Physiol Regul Integr Comp Physiol. 291, R796–802 (2006). [DOI] [PubMed] [Google Scholar]
- Koike H., Iijima M. & Chaki S. Involvement of the mammalian target of rapamycin signaling in the antidepressant-like effect of group II metabotropic glutamate receptor antagonists. Neuropharmacology. 61, 1419–23 (2011). [DOI] [PubMed] [Google Scholar]