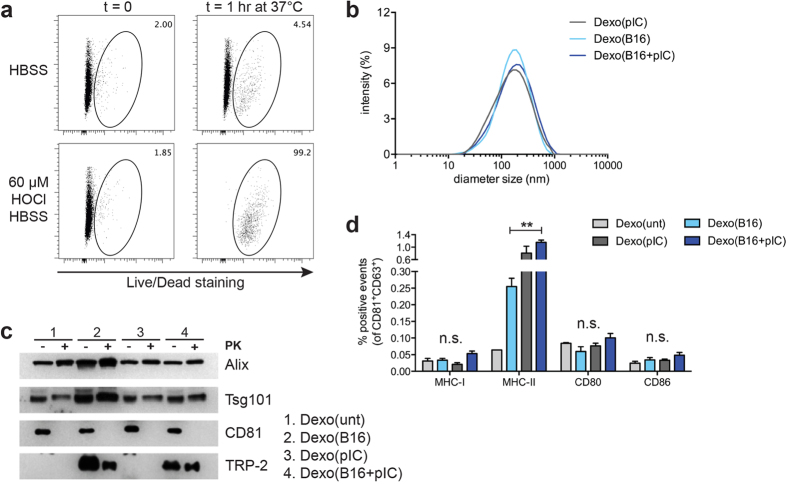
Figure 4. HOCl-oxidized B16-F10 melanoma cells can be used as a source of tumor antigens for the production of DC exosomes containing melanoma-derived epitopes.
(a) B16-F10 melanoma cells were resuspended in 60 μM HOCl HBSS buffer and incubated at 37 °C for 1 hr to induce oxidation of the tumor cells. After incubation, cells were stained with a viability dye and analyzed by flow cytometry. As controls, viability of B16-F10 cells before incubation (t = 0) or B16-F10 cells resuspended in HBSS buffer was also analyzed. Numbers indicate the frequency of dead cells gated in the total population of B16-F10 cells. (b) Dexo were purified from the supernatant of DCs preincubated with oxidized B16-F10 obtained as in (a) with or without poly(I:C) (Dexo(B16 + pIC) and Dexo(B16), respectively) or with poly(I:C) only as a control (Dexo(pIC)) following the described protocol for exosomes isolation. After purification, the size of Dexo was measured by DLS analysis. (c) Presence of the exosome-specific markers Alix (100 kDa) and Tsg101 (46 kDa) (intravesicular) and CD81 (26 KDa) (vesicle membrane) and of the full-length melanoma-derived protein TRP-2 (59 kDa) was detected by western blot analysis of Dexo(unt), Dexo(B16), Dexo(pIC) and Dexo(B16 + pIC) digested or not with proteinase K to confirm purification of bona-fide exosomes and exosomal localization of the antigens. (d) Surface staining for MHC-I, MHC-II, CD80 and CD86 and flow cytometric analysis of Dexo(unt), Dexo(B16), Dexo(pIC) and Dexo(B16 + pIC). Percentages represent the frequency of positive events among CD81+ CD63+ particles. Data in (d) represent mean ± SEM from 2 independent experiments (N = 6). Statistical analysis was performed by one-way ANOVA and Bonferroni post-hoc test correction to compare Dexo(B16), Dexo(pIC) and Dexo(B16 + pIC). **P < 0.01 and n.s. = not significant.