Table 2.
Amination of heteroarenes and arenes.[a]
| ||||
|---|---|---|---|---|
| Entry | 1 | t [h][b] | 3 | Yield [%][c] |
| 1 |
|
1; 5 |
|
96 |
| 2 | 1; 5 | 93 | ||
| 3 | 1; 5 | 95 | ||
| 4 |
|
1;[d] 5 |
|
71 |
| 5 | 1;[d] 5 | 70 | ||
| 6 |
|
1; 12 |
|
82 |
| 7 | 1; 12 | 92 | ||
| 8 | 1; 12 | 95 | ||
| 9 |
|
1; 24 |
|
96 |
| 10 |
|
1; 5 |
|
85 |
| 11 |
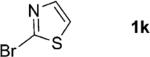
|
1; 5 |
|
90 |
| 12 |
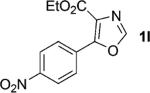
|
1; 4 |
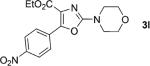
|
89 |
| 13 |
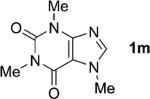
|
1;[e] 24 |
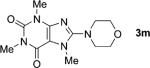
|
82 |
| 14 |
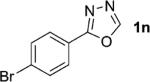
|
1; 4 |
|
91 |
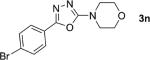
| 15 |
|
2; 12[f] |
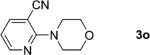
|
81 |
| 16 |

|
2; 12[f] |
|
79 |
| 17 |
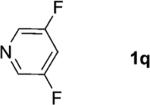
|
2; 12[f] |
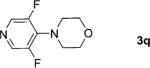
|
91 |
| 18 |
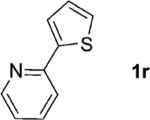
|
2; 12[f] |
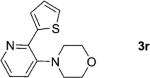
|
74 |
| 19 |
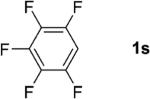
|
1; 12 |
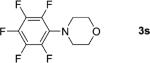
|
75 |
Reactions typically run on 0.2 mmol scale. 1 (2.1 equiv), 2 (1.0 equiv, 0.08 m), Zn(tmp)2 (1.0 equiv).
Reaction time for deprotonation and amination step respectively.
Yield of isolated product.
Zn-(tmp)2·LiCl·MgCl2[4g] was used as base to form stable zincate intermediates.
Deprotonation step run in CH2Cl2 because of the poor solubility of 1m in THF.
Amination step run at 50°C.
