Abstract
Both the American Academy of Pediatrics (AAP) and the Institute of Medicine (IOM) recommend delaying the introduction of cow’s milk until after 1 year of age due to its low absorbable iron content. We used a novel computerized decision support system to gather data from multiple general pediatrics offices. We asked families whether their child received cow’s milk before one year of age, had a low iron diet, or used low iron formula. Then, at subsequent visits, we performed a modified developmental assessment using the Denver II. We assessed the effect of early cow’s milk or a low iron diet on the later failure of achieving developmental milestones. We controlled for covariates using logistic regression. Early cow’s milk introduction (OR 1.30, p = 0.012), as well as a low iron diet or low iron formula (OR 1.42, p < 0.001), were associated with increased rates of milestone failure. Only personal-social milestones (OR 1.44, p = 0.002) showed a significantly higher rate of milestone failure. Both personal-social (OR 1.42, p < 0.001) and language (OR 1.22, p = 0.009) showed higher rates of failure in children with a low iron diet.
Conclusions
There is an association between the introduction of cow’s milk before one year of age and the rate of delayed developmental milestones after one year of age. This adds strength to the recommendations from the AAP and IOM to delay cow’s milk introduction until after one year of age.
BACKGROUND
The American Academy of Pediatrics (AAP) recommends in a 2010 Clinical Report that cow’s milk only be introduced as a main drink after a child reaches 12 months of age.[4] Furthermore, in the absence of another source of dietary iron, the iron content in cow’s milk simply does not meet the baseline requirements for dietary iron defined by the Institute of Medicine (IOM).[11] Support for these recommendations comes from classic and oft-cited studies suggesting that early cow’s milk introduction is associated with the development of iron deficiency anemia.[17,18,21] More recently, indirect evidence on a larger scale shows a correlation between the decline in cow’s milk introduction and the decline in iron deficiency anemia,[7] which also supports this relationship. While the AAP discourages cow’s milk as a main drink before 12 months, they support introduction of other dairy products (such as yogurt) from 6 months of age. A summary of the macro- and micronutrient content of cow’s milk are shown in Table 1.
Table 1.
Nutritional Content of Vitamin D Fortified Cow’s Milk[22]
| Nutrient | Unit | Amount |
|---|---|---|
| Water | g | 88.13 |
| Energy | kcal | 61 |
| Protein | g | 3.15 |
| Total lipid (fat) | g | 3.25 |
| Carbohydrate, by difference | g | 4.8 |
| Fiber, total dietary | g | 0 |
| Sugars, total | g | 5.05 |
| Minerals | ||
| Calcium, Ca | mg | 113 |
| Iron, Fe | mg | 0.03 |
| Magnesium, Mg | mg | 10 |
| Phosphorus, P | mg | 84 |
| Potassium, K | mg | 132 |
| Sodium, Na | mg | 43 |
| Zinc, Zn | mg | 0.37 |
| Vitamins | ||
| Vitamin C, total ascorbic acid | mg | 0 |
| Thiamin | mg | 0.046 |
| Riboflavin | mg | 0.169 |
| Niacin | mg | 0.089 |
| Vitamin B-6 | mg | 0.036 |
| Folate, DFE | Âμg | 5 |
| Vitamin B-12 | Âμg | 0.45 |
| Vitamin A, RAE | Âμg | 46 |
| Vitamin A, IU | IU | 162 |
| Vitamin E (alpha-tocopherol) | mg | 0.07 |
| Vitamin D (D2 + D3) | Âμg | 1.3 |
| Vitamin D | IU | 51 |
| Vitamin K (phylloquinone) | Âμg | 0.3 |
| Lipids | ||
| Fatty acids, total saturated | g | 1.865 |
| Fatty acids, total monounsaturated | g | 0.812 |
| Fatty acids, total polyunsaturated | g | 0.195 |
| Cholesterol | mg | 10 |
| Other | ||
| Caffeine | mg | 0 |
Most evidence for the association between cow’s milk introduction and the development of significant neurodevelopmental delay is indirect or weak, and there are no large, prospective studies to guide the creation of dietary recommendations from pediatricians to parents parents. Recent data have shown that delayed introduction of cow’s milk protein (either in cow’s milk or cow’s milk infant formula) may increase the risk for eczema after 2 years of age,[19] so the ideal timing of introduction of cow’s milk containing foods remains controversial.
Chronic anemia has long been associated with developmental delay,[12,14] and this effect may persist even after correction with iron therapy.[13] More recently, the presence of iron deficiency alone, even without anemia, has been associated with neurocognitive delays later in life;[1] however, patients with both anemia and iron-deficiency tend to have more severe delay than those with iron-deficiency alone.[1] Recent data show that the issue may be more complex than previously thought, as one long term followup study showed worse neurological outcomes (specifically for spatial memory and visual-motor integration) for children on iron-fortified formulas.[15]
Since the overall goal of iron deficiency prevention is to avoid negative neurodevelopmental outcomes, we sought to determine the relationship between this widespread dietary recommendation and the outcome of interest: failed developmental milestones. We analyzed longitudinal data collected using the Child Health Improvement through Computer Automation (CHICA) system to assess the association between cow’s milk introduction before one year of age and failed developmental milestones after one year of age. This is the first study to our knowledge that shows a direct relationship between early cow’s milk introduction and subsequent delayed developmental milestones.
METHODS
The CHICA System
The CHICA system was created in 2002, and has seen continuous clinical operation since 2004. CHICA is currently installed in four pediatric outpatient clinics in the Indianapolis metropolitan area. Over 88,000 total pediatric encounters in more than 32,000 patients have been recorded to date. The system consists of the use of a pre-screener form (PSF), which is given to family members in the waiting room, as well as a physician worksheet (PWS), which is automatically created for use by the physician. Logic rules are applied to a comprehensive set of pediatric preventative care guidelines, which use patient demographics, data from the PSF, information from previous patient visits, and the electronic medical record to determine suitability of a wide range of interventions, which are delivered to the provider via the PWS through physician reminders. This system has seen extensive use studying a variety of problems.[2,3,5,6,8]
For this study, we utilized two sets of questions in the CHICA database (Fig. 1). All patients who were followed for sufficient time to have answers to both sets of questions were included in the analysis. The first set of questions is taken from the PSF and answered directly by parents: “Have you given <child’s name> cow’s milk to drink?” This question is only asked for visits occurring between 0–12 months of age. We used this question to determine whether a child had early cow’s milk exposure, and then codified the presence or absence of this trait. We also obtained a question from the PSF related to dietary iron: "Does <child’s name> get fed two or more servings of the following foods daily: iron fortified cereals, fruits, vegetables, juices, or pureed meats?" and “Does <child’s name> drink low iron formula?” and then codified the presence or absence of this trait as a variable. The United States Food and Drug Administration (FDA) defines low iron formula as that containing less than 6.7 mg/L of elemental iron, and these account for up to 30% of elective (non-government-supplied) formula consumption. In most cases, low iron formula is chosen due to concerns for gastrointestinal complaints or other symptoms, and is rarely recommended by pediatricians. Furthermore, there are significant worldwide differences in average formula iron content: the average iron content in iron-fortified infant formulas in Europe and other countries tends to be less than in the United States (4–7 mg/L vs. 12–13 mg/L).[4,15,16] Patients with a negative response to the iron-containing foods question or an affirmative response to the low iron formula question were coded as true for a low-iron diet before 12 months of age.
Figure 1. Examples of Pre-Screener Form (PSF) questions from the CHICA system used to gather study data.
The whole form is shown on the left of the figure, while a detail view of each question is shown at right. Figure 1A: Example of the PSF question on milk introduction given to the family. Figure 1B: Example of the PSF question on low iron diet. Figure 1C: Example of the PSF developmental assessment. This example is for a 7 month old; developmental questions are age-specific.
The second set of questions was present on both the PSF and PWS, and thus answerable by either the parent or physician, but always confirmed by the physician. Therefore, the screening prompt for developmental delay in each category could be recognized by either parent or physician, but would not be entered into the system unless verified by the physician. Each type of four developmental categories (personal social, language, fine motor adaptive, and gross motor) was assessed using a series of specific questions from the Denver Developmental II milestones specific for a patient’s age.[9] The parent or physician is given a short list of milestones appropriate for that patient’s age, and if a child is unable to perform the relevant milestone, that developmental category is marked as failed for that visit. Only responses after 12 months of age were used. If a child had any failure of a developmental milestone after 12 months, we coded the presence or absence of any failure as a variable. We also coded variables for each developmental category for separate analysis.
For this study, we assessed all patients in the CHICA system with both a response to the cow’s milk question indicating early introduction (or lack of introduction) of milk, and a response to the developmental screen indicating failure (or passing) of four developmental categories. We also obtained demographic data for gender, race, clinic location, and language preference (as denoted by the language of the parent-completed PSF) for use in analyses.
We did not measure iron levels directly for this study. A small minority of patients in our database had iron levels drawn and present in their medical record. However, since our decision support system was not designed to directly measure iron levels, since it is not part of routine care, there were insufficient patients in our database with iron levels to evaluate the effect of early milk introduction
Statistical Analysis
We built a logistic regression model to control for other variables that may affect the rate of developmental delay, such as demographic variables and the presence of a low iron diet. Our dependent variable was the presence or absence of a failed developmental milestone in a visit after 12 months of age (both in aggregate, and as a separate regression for each of the four developmental categories). Independent variables included in the model were: gender, race, insurance type, clinic location (one of four sites using the CHICA system), language, the presence of cow’s milk in the diet before 12 months of age, and the presence of a low iron diet or low iron formula before 12 months of age. Models were built using the R software package (http://www.r-project.org), using the generalized linear model (glm) function, with a binomial link function, and the model parameters were computed using maximum likelihood estimation. Models were compared using the Akaike Information Criterion (AIC). We considered a p-value of 0.05 or less to be statistically significant.
The Institutional Review Board at Indiana University reviewed and approved this study prior to data acquisition or analysis.
RESULTS
We found 7110 patients between 2005 and 2012 in our database who met our inclusion criteria: at least one visit before 12 months with an answer to cow’s milk introduction / dietary iron, and at least one visit after 12 months with a developmental assessment.
Study demographics (Table 2) reflect the clinic population where the CHICA system is installed. There were slightly more males (51.4%) than females. The majority of patients were either Black (38.6%) or Hispanic (36.6%). A majority of patients were on Medicaid (80.2%), and few had commercial insurance (3.2%). Most families used English (53.3%) as their primary language, although a large minority spoke Spanish (20.2%). Most patients were seen at the largest primary care center using CHICA (78.7%), and the remaining patients were divided somewhat equally among three other sites using the system.
Table 2.
Subject Demographics
| Total Patients | 7110 |
|---|---|
| Gender | |
| Male | 3659 (51.4%) |
| Race | |
| White | 846 (11.9%) |
| Black | 2745 (38.6%) |
| Hispanic | 2602 (36.6%) |
| Asian | 115 (1.6%) |
| American Indian | 10 (0.14%) |
| Other | 792 (11.1%) |
| Insurance Type | |
| Medicaid | 5703 (80.2%) |
| Commercial | 228 (3.2%) |
| Self Pay | 506 (7.1%) |
| Other | 56 (0.79%) |
| Unknown | 617 (8.7%) |
| Language | |
| English | 3794 (53.3%) |
| Spanish | 1434 (20.2%) |
| Unknown / Other | 1882 (26.5%) |
| Clinic | |
| Clinic 1 | 5596 (78.7%) |
| Clinic 2 | 367 (5.2%) |
| Clinic 3 | 528 (7.4%) |
| Clinic 4 | 619 (8.7%) |
| Low Iron Diet or Low Iron Formula | 4100 (57.7%) |
| Early Milk Introduction | 576 (8.0%) |
| Any Milestone Failure | 1514 (21.3%) |
| Language | 847 (11.9%) |
| Personal Social | 972 (13.7% |
| Fine Motor Adaptive | 67 (0.94%) |
| Gross Motor | 177 (2.5%) |
There was some overlap of patients with low iron and early milk introduction. 334 / 576 (58.0%) of patients with early milk introduction before 12 months also had a low iron diet before 12 months, although this is similar to the rate of low iron diet among all subjects we assessed: 4100 / 7110 (57.7%).
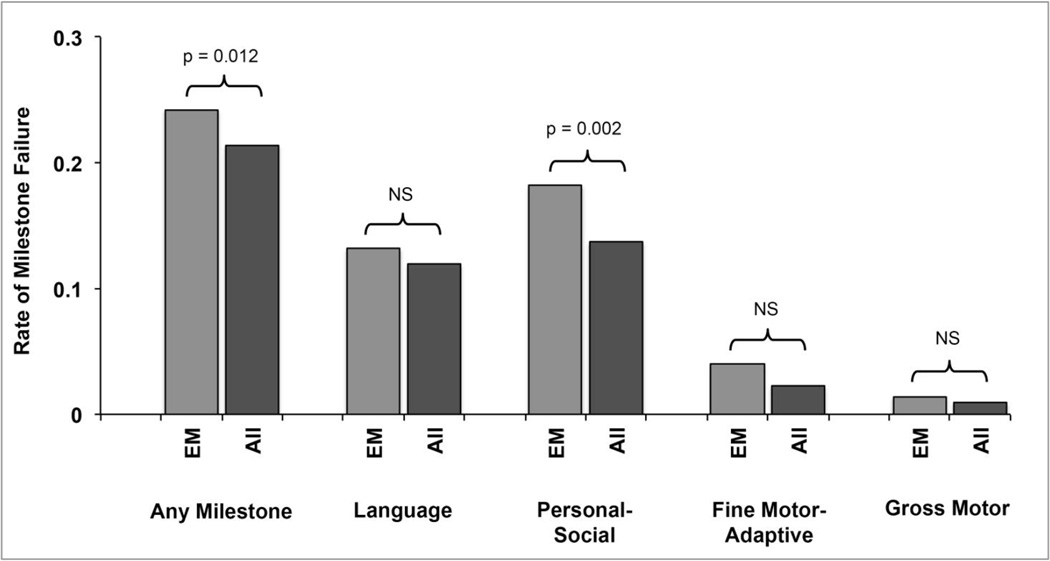
Milk introduction before one year of age resulted in a significantly increased rate of failure of some, but not all, developmental milestones after one year of age (Fig. 2). Children who had introduction of cow’s milk before one year of age were 30% more likely to have failed developmental milestones after one year of age, after controlling for covariates (OR 1.30, p = 0.012). When we analyzed each developmental category separately, we found that only personal-social development was significantly influenced by early cow’s milk introduction after controlling for covariates (OR 1.44, p = 0.002). There was an increased rate of developmental failure for each of the other three developmental categories, but none achieved significance at the p < 0.05 level.
Figure 2. Rate of milestone failure after 1 year of age for early milk drinkers.
There was a significant difference found for the rate of failure of any milestone (p = 0.012) and for personal-social milestones (p = 0.002), but the remaining developmental categories were non-significant. EM = Early Milk. NS = Non-significant.
Low iron diet in the form of low iron formula or lack of iron-containing foods also resulted in significantly increased rate of failed developmental milestones after one year of age (Fig. 3). Children who had a low iron diet or a low iron formula before one year of age were 42% more likely to have failed developmental milestones after one year of age after controlling for covariates (OR 1.42, p < 0.001). When we analyzed each developmental category separately, we found that language (OR 1.22, p = 0.009) and personal-social (OR 1.42, p < 0.001) development was significantly influenced by low iron diet or low iron formula after controlling for covariates. There was an increased rate of developmental failure for each of the other two developmental categories, but neither achieved significance at the p < 0.05 level.
Figure 3. Rate of milestone failure after 1 year of age for children with a low iron diet.
There was a significant difference found for the rate of failure of any milestone (p < 0.001), language milestones (p = 0.009) and for personal-social milestones (p < 0.001), but the remaining developmental categories were non-significant. LID = Low Iron Diet or Low Iron Formula. NS = Non-significant.
COMMENT
We found an association between the early introduction of cow’s milk, and the later delay of milestones. We found an equivalent association for children with the intake of low iron formula or consumption of a low iron diet before one year of age. We used a large observational cohort of patients followed longitudinally in a group of general pediatrics offices.
Interestingly, only one category in the Denver II was influenced by early cow’s milk introduction: personal-social development. There were more patients with language or personal-social delay in our cohort (11.9 and 13.7%, respectively), and fewer with fine motor or gross motor delay (0.9 and 2.5%, respectively). This may reflect a variety of effects, although given the data collected by our study, we can only speculate as to the cause. First, milk introduction may be associated with developmental delay via its effect on dietary iron. This would most likely be secondary to milk’s effect on iron absorption, as there is a theoretic effect of calcium and casein in cow’s milk on the absorption of other sources of dietary iron.[10,20,23]
Second, cow’s milk may have a separate mechanism contributing to developmental delay, such as the influence of a proteinaceous component of milk (hormones, allergens, etc). Finally, the use of milk before one year of age may simply reflect poor parental compliance with a pediatrician’s recommendations, and as such could be a correlate of other behaviors influencing child development. Unfortunately, our study was not able to assess the mechanism for the observed association.
Although after controlling for confounders, the early introduction of cow's milk as main drink was still strongly associated with poor neurodevelopmental outcome, there may be other nutritional (e.g. intake of essential fatty acids) and non nutritional factors (e.g. gestational age, educational level of mother, etc.) that may have impacted on the results. These are largely outside the scope of our data.
A weakness in our study is the lack of a direct measurement of iron deficiency or measurement of hemoglobin levels linked with our clinical data. However, we would expect a minority of patients seen by general pediatricians to have an iron level drawn as part of routine care, so these data are not prevalent enough in our study population to make this assessment. Hemoglobin levels were not drawn routinely (office point of care hematocrit was used in many patients at one year of age, but these data were not collected as part of CHICA). Also, many patients do not have data beyond three years of age, so later development of milestone delay (or resolution of previous delay) may not yet be apparent in our cohort. Therefore, we do not know if the association we observed is persistent. We do provide indirect evidence of the role of iron deficiency in early milk drinkers, since there is a very similar effect on failed milestones for early low iron intake. Furthermore, there may be other unmeasured confounders which we did not collect, but which may affect developmental delay, such as: gestational age, birth weight, mother's diabetic status, duration of breast feeding, use of iron supplements, overall growth status, mother's education level, and maternal age. We did not assess for supplemental sources of iron, such as iron drops in infants. In an effort to maintain simplicity, we also did not quantify the amount of cow’s milk that was introduced, simply whether it had been introduced at all.
We would suggest that physicians continue to follow the AAP and IOM recommendations to delay cow’s milk introduction due to inadequate iron content for children less than one year of age. We have shown using a large patient cohort that the early introduction of cow’s milk is associated with increased rates of personal-social developmental delay.
ACKNOWLEDGEMENTS
We thank Htaw Htoo, Tammy Dugan, and Ashley Street for their help in data acquisition and management of the CHICA database, as well as other members of the Child Health Improvement Research and Development Laboratory (CHIRDL). This study was not grant supported, although the CHICA system receives support from the following NIH grants: R01DK092717, R01HS017939, R01HS018453, R01HS020640.
Footnotes
All authors report that they have no conflict of interest, financial or otherwise, related to the production of this manuscript.
References
- 1.Akman M, Cebeci D, Okur V, Angin H, Abali O, Akman AC. The effects of iron deficiency on infants' developmental test performance. Acta Paediatr. 2004;93:1391–1396. [PubMed] [Google Scholar]
- 2.Anand V, Biondich PG, Liu G, Rosenman M, Downs SM. Child Health Improvement through Computer Automation: the CHICA system. Studies in health technology and informatics. 2004;107:187–191. [PubMed] [Google Scholar]
- 3.Anand V, Downs SM. Probabilistic asthma case finding: a noisy or reformulation. AMIA Annual Symposium proceedings / AMIA Symposium AMIA Symposium. 2008:6–10. [PMC free article] [PubMed] [Google Scholar]
- 4.Baker RD, Greer FR Committee on Nutrition American Academy of P. Diagnosis and prevention of iron deficiency and iron-deficiency anemia in infants and young children (0–3 years of age) Pediatrics. 2010;126:1040–1050. doi: 10.1542/peds.2010-2576. [DOI] [PubMed] [Google Scholar]
- 5.Biondich PG, Downs SM, Anand V, Carroll AE. Automating the recognition and prioritization of needed preventive services: early results from the CHICA system. AMIA Annual Symposium proceedings / AMIA Symposium AMIA Symposium. 2005:51–55. [PMC free article] [PubMed] [Google Scholar]
- 6.Carroll AE, Biondich PG, Anand V, Dugan TM, Sheley ME, Xu SZ, Downs SM. Targeted screening for pediatric conditions with the CHICA system. Journal of the American Medical Informatics Association : JAMIA. 2011;18:485–490. doi: 10.1136/amiajnl-2011-000088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cusick SE, Mei Z, Freedman DS, Looker AC, Ogden CL, Gunter E, Cogswell ME. Unexplained decline in the prevalence of anemia among US children and women between 1988–1994 and 1999–2002. The American journal of clinical nutrition. 2008;88:1611–1617. doi: 10.3945/ajcn.2008.25926. [DOI] [PubMed] [Google Scholar]
- 8.Downs SM, Zhu V, Anand V, Biondich PG, Carroll AE. The CHICA smoking cessation system. AMIA Annual Symposium proceedings / AMIA Symposium AMIA Symposium. 2008:166–170. [PMC free article] [PubMed] [Google Scholar]
- 9.Frankenburg WK, Dodds JB. The Denver developmental screening test. The Journal of pediatrics. 1967;71:181–191. doi: 10.1016/s0022-3476(67)80070-2. [DOI] [PubMed] [Google Scholar]
- 10.Heath AL, Fairweather-Tait SJ. Clinical implications of changes in the modern diet: iron intake, absorption and status. Best practice & research Clinical haematology. 2002;15:225–241. [PubMed] [Google Scholar]
- 11.Institute of Medicine (U.S.) Panel on Micronutrients. DRI : dietary reference intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc : a report of the Panel on Micronutrients … and the Standing Committee on the Scientific Evaluation of Dietary Reference Intakes, Food and Nutrition Board, Institute of Medicine. Washington D.C.: National Academy Press; 2001. [Google Scholar]
- 12.Lozoff B, Brittenham GM, Wolf AW, McClish DK, Kuhnert PM, Jimenez E, Jimenez R, Mora LA, Gomez I, Krauskoph D. Iron deficiency anemia and iron therapy effects on infant developmental test performance. Pediatrics. 1987;79:981–995. [PubMed] [Google Scholar]
- 13.Lozoff B, Jimenez E, Hagen J, Mollen E, Wolf AW. Poorer behavioral and developmental outcome more than 10 years after treatment for iron deficiency in infancy. Pediatrics. 2000;105:E51. doi: 10.1542/peds.105.4.e51. [DOI] [PubMed] [Google Scholar]
- 14.Lozoff B, De Andraca I, Castillo M, Smith JB, Walter T, Pino P. Behavioral and developmental effects of preventing iron-deficiency anemia in healthy full-term infants. Pediatrics. 2003;112:846–854. [PubMed] [Google Scholar]
- 15.Lozoff B, Castillo M, Clark KM, Smith JB. Iron-fortified vs low-iron infant formula: Developmental outcome at 10 years. Archives of Pediatrics & Adolescent Medicine. 2012;166:208–215. doi: 10.1001/archpediatrics.2011.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moy RJ. Iron fortification of infant formula. Nutrition research reviews. 2000;13:215–227. doi: 10.1079/095442200108729070. [DOI] [PubMed] [Google Scholar]
- 17.Pizarro F, Yip R, Dallman PR, Olivares M, Hertrampf E, Walter T. Iron status with different infant feeding regimens: relevance to screening and prevention of iron deficiency. The Journal of pediatrics. 1991;118:687–692. doi: 10.1016/s0022-3476(05)80027-7. [DOI] [PubMed] [Google Scholar]
- 18.Sadowitz PD, Oski FA. Iron status and infant feeding practices in an urban ambulatory center. Pediatrics. 1983;72:33–36. [PubMed] [Google Scholar]
- 19.Snijders BE, Thijs C, van Ree R, van den Brandt PA. Age at first introduction of cow milk products and other food products in relation to infant atopic manifestations in the first 2 years of life: the KOALA Birth Cohort Study. Pediatrics. 2008;122:e115–e122. doi: 10.1542/peds.2007-1651. [DOI] [PubMed] [Google Scholar]
- 20.Thompson BA, Sharp PA, Elliott R, Fairweather-Tait SJ. Inhibitory effect of calcium on non-heme iron absorption may be related to translocation of DMT-1 at the apical membrane of enterocytes. Journal of agricultural and food chemistry. 2010;58:8414–8417. doi: 10.1021/jf101388z. [DOI] [PubMed] [Google Scholar]
- 21.Tunnessen WW, Jr, Oski FA. Consequences of starting whole cow milk at 6 months of age. The Journal of pediatrics. 1987;111:813–816. doi: 10.1016/s0022-3476(87)80193-2. [DOI] [PubMed] [Google Scholar]
- 22.U.S.D.A. [Accessed November 5th, 2013];United States Department of Agriculture Nutrient Data Laboratory. 2013 http://www.ars.usda.gov/main/site_main.htm?modecode=12-35-45-00.
- 23.Ziegler EE. Consumption of cow's milk as a cause of iron deficiency in infants and toddlers. Nutrition reviews. 2011;69(Suppl 1):S37–S42. doi: 10.1111/j.1753-4887.2011.00431.x. [DOI] [PubMed] [Google Scholar]