Abstract
Background
Primary Sjögren’s syndrome (pSS) is one of the most common chronic systemic autoimmune diseases, and thrombocytopenia is one of the hematological manifestations of pSS. When platelet and endothelial cells are activated, P-selectin is expressed on the cell surface. This study aimed to investigate the role of P-selectin autoantibodies in the pathogenesis of thrombocytopenia in pSS.
Material/Methods
P-selectin autoantibodies were measured by enzyme-linked immunosorbent assay (ELISA) in 38 pSS patients without thrombocytopenia and 32 pSS patients with thrombocytopenia, 32 idiopathic thrombocytopenic purpura (ITP) patients, and 35 healthy controls.
Results
The plasma P-selectin autoantibodies (A490) in ITP patients and pSS patients with/without thrombocytopenia were significantly higher than those in healthy controls, but there were no significant differences between ITP patients and pSS patients with thrombocytopenia. The positive rate of P-selectin autoantibodies in pSS patients with thrombocytopenia was significantly higher than that in ITP patients. The platelet count was lower in P-selectin autoantibodies-positive patients, while among pSS patients with thrombocytopenia, the platelet count was lower in P-selectin autoantibodies-positive patients than in P-selectin autoantibodies-negative patients. In ITP patients and pSS patients with thrombocytopenia, the platelet count was lower in P-selectin autoantibodies-positive patients.
Conclusions
Elevated plasma P-selectin autoantibodies may play a role in the pathogenesis of thrombocytopenia in pSS patients.
MeSH Keywords: Autoantibodies, P-Selectin; Endothelial Cells; Purpura, Thrombocytopenic, Idiopathic; Sjögren’s Syndrome, Primary; Thrombocytopenia
Background
Primary Sjögren’s syndrome (pSS) is one of the most common chronic systemic autoimmune diseases. It primarily affects the salivary and lachrymal glands, and is characterized by the classical symptoms of oral and ocular dryness [1,2], along with widespread pain and intense fatigue. However, a significant proportion of patients develop extraglandular systemic manifestations involving several organs (e.g., central nervous system, lung, liver, and kidney). pSS has been estimated to affect 0.01–0.1% of the general population [3]. Secondary SS (sSS) is usually associated with other rheumatic conditions, such as rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE), of which the most common is RA [4–6]. As a systemic autoimmune disease, B cells play an important role in the pathogenesis of pSS [7]. There are several autoantibodies present during pSS [8], and antinuclear antibodies (ANA) are the most common, detectable in up to 80% of pSS patients. However, the most specific autoantibodies in pSS are the intracellular antigens Ro52/TRIM21, Ro60/TROVE2, and La/SSB ribonucleoproteins [9]. These autoantibodies are detectable in patients several years before the first clinical manifestation of pSS, and may be used to predict the disease [10]. Rheumatoid factors (RF) are also frequently found in these patients, and are often associated with higher disease activity [8]. Hematological manifestations are also noted in pSS patients and include amenia, leukopenia, and thrombocytopenia [11]. Previous studies have reported that thrombocytopenia is present in 5–7% of pSS patients [12]; it is an isolated manifestation or a component of pancytopenia, but life-threatening, severe thrombocytopenia is very rare.
The cause of thrombocytopenia in pSS patients is still poorly understood. It has been suggested that thrombocytopenia in pSS involves a humoral autoimmune mechanism in which an antibody produced is able to bind to the cell membrane [13]. Idiopathic thrombocytopenic purpura (ITP) is also an autoimmune disorder characterized by thrombocytopenia [14]. It has been demonstrated that platelet autoantibodies are detectable in ITP patients. Platelet autoantibodies often recognize platelet glycoproteins (GP) such as GPIb, GP IX, GPIIb/IIIa, and P-selectin [15,16]. Platelet-derived microparticles could highlight platelet activation in pSS [17]. P-selectin is a member of the selectin family of cell-adhesion molecules, and may modulate the interaction of leukocytes and platelets with the endothelium [18]. P-selectin is stored in the granules of endothelial cells and platelets; it is expressed on their surface after activation, and shed into the plasma in the soluble form. P-selectin plays an important role in thrombosis and prothrombotic states [19–22]. P-selectin autoantibodies may affect the functions of platelets and endothelial cells. pSS is a chronic inflammatory systemic autoimmune disease with endothelial injury.
In the present study, enzyme-linked immunosorbent assay (ELISA) was used to detect the plasma P-selectin autoantibodies, and the role of P-selectin autoantibodies in the thrombocytopenia of pSS patients was evaluated. To the best of our knowledge, this is the first study to measure P-selectin autoantibodies in pSS patients.
Material and Methods
Patients and healthy controls
A retrospective study was conducted from July 1, 2012, to July 1, 2014. Seventy pSS patients were treated in our department and pSS was diagnosed according to the criteria of the American–European Consensus Group for pSS in 2002 [23]. Patients with secondary Sjögren’s syndrome (sSS) with any other autoimmune diseases, such as RA, SLE, and other connective tissue diseases, were excluded from this study. Of the 70 pSS patients, 32 pSS patients had thrombocytopenia, and 38 pSS patients had no thrombocytopenia. In addition, ITP patients (n=32) were also enrolled as controls and ITP was diagnosed according to the criteria for ITP [24]. Moreover, 35 healthy subjects were recruited as healthy controls. This study was approved by the Ethics Committee of The First Affiliated Hospital of Soochow University, and all participants gave their written informed consent before study. The plasma samples were collected from all the subjects and stored at −80°C until use.
Detection of plasma P-selectin autoantibodies
We pre-coated 96-well Microtiter plates with goat anti-mouse polyclonal antibody at 5 μg/μL (100 μL/well) at 4°C overnight. These plates were washed 3 times with phosphate-buffered saline (PBS) containing 0.05% Tween 20 (PBS-T), and treated with blocking buffer (1% bovine serum albumin [BSA] in PBS-T) at 4°C overnight. After washing 3 times, P-selectin monoclonal antibody at 10 μg/ml was added to the plates followed by incubation overnight at 4°C. The plates were blocked on the second day, and then washed for use. We added 1×109/ml lysed platelets diluted at 1: 5 to the plates (100 μl/well) followed by incubation at 37°C for 2 h. After washing with PBS-T, the diluted plasma samples (1:5, 100 μl/well) were added, followed by incubation at 37°C for 1.5 h. After washing 3 times, 100 μl of rabbit anti-human IgG conjugated to horseradish peroxidase was added to each well followed by incubation at 37°C for 1 h. After washing 6 times, TMB was added and incubated for 10 min, and the reaction was stopped by addition of 3M sulfuric acid. Then, the optical density (OD) was measured at 490 nm with a microplate reader. All measurements were performed in duplicate. The mean OD +2 fold of standard deviation from the plasma samples of healthy controls was considered as the upper limit of normal. It was defined as positive if mean OD was higher than the upper limit of normal; otherwise, it was defined as negative.
Blood platelet counting
Blood platelet count was determined with an XE-5000 multiparameter automatic hematology analyzer (Sysmex, Kobe, Japan).
Statistical analysis
Statistical analysis was done with SPSS version 18.0 for Windows. Data are presented as means ± standard deviation (SD). Data were compared with t-test between 2 groups. A value of P<0.05 was considered statistically significant.
Results
Patient characteristics
A total of 102 patients (13 males and 89 females) and 35 healthy controls (18 males and 17 females) were enrolled into the present study between 2012 and 2014. There were 70 pSS patients (6 males and 64 females) and 32 ITP patients (7 males and 25 females). Of the 70 pSS patients, 38 had no thrombocytopenia (4 males and 34 females) and the other 32 had concomitant thrombocytopenia (2 males and 30 females). The demographic characteristics of subjects included are summarized in Table 1. There were no significant differences in sex and age between pSS patients with thrombocytopenia and ITP patients. We analyzed the platelet count of patients and normal controls (Figure 1). The platelet count in ITP patients and pSS patients with thrombocytopenia (35.7±22.6×109/L and 27.5±11.1×109/L, respectively) was lower than that in healthy controls (157.3±52.5×109/L) (P=0.000 and P=0.000, respectively), and there was no significant difference in the platelet count between pSS patients without thrombocytopenia (181.6±49.1×109/L) and healthy controls. There was no significant difference in the platelet count between pSS patients with thrombocytopenia and ITP controls (P=0.072).
Table 1.
Demographic features of subjects enrolled in this study.
| Number | Gender, n (%) | Median age, y (range) | ||
|---|---|---|---|---|
| Male | Female | |||
| pSS | 70 | 6 (8.6) | 64 (91.4) | 42 (20–60) |
| Without thrombocytopenia | 38 | 4 (10.5) | 34 (89.5) | 43 (20–53) |
| With thrombocytopenia | 32 | 2 (6.3) | 30 (93.7) | 42 (23–60) |
| ITP | 32 | 7 (21.9) | 25 (78.1) | 36 (19–54) |
| Normal control | 35 | 18 (51.4) | 17 (48.6) | 39 (18–59) |
Figure 1.
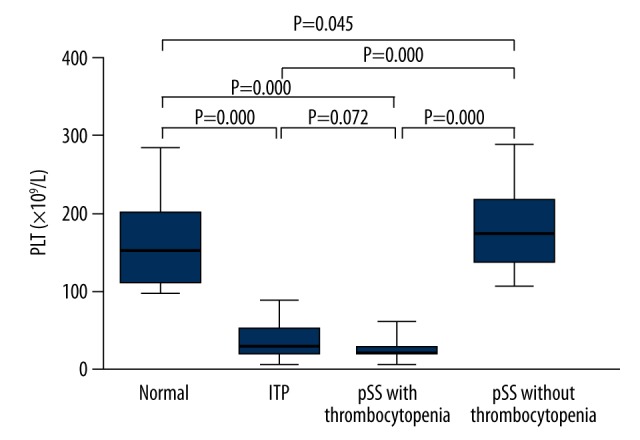
Platelet count in ITP patients, pSS patients with/without thrombocytopenia and healthy controls. Data are expressed as mean ±SD. There was a lower platelet count in ITP patients and pSS patients with thrombocytopenia than in healthy controls (P=0.000 and P=0.000, respectively), and no marked difference in the platelet count was observed between pSS patients with/without thrombocytopenia and healthy controls (P=0.072 and P=0.045, respectively).
Plasma P-selectin autoantibodies
P-selectin autoantibodies were measured in pSS patients with/without thrombocytopenia, ITP patients, and healthy controls (Figure 2). The plasma P-selectin autoantibodies in ITP patients, pSS patients with thrombocytopenia, and pSS patients without thrombocytopenia (0.796±0.097, 0.854±0.142, and 0.738±0.090, respectively) was significantly higher than that in healthy controls (0.684±0.110) (P=0.000, P=0.000, and P=0.024, respectively), but there was no significant difference in the P-selectin autoantibodies between ITP patients and pSS patients with thrombocytopenia (P=0.062).
Figure 2.
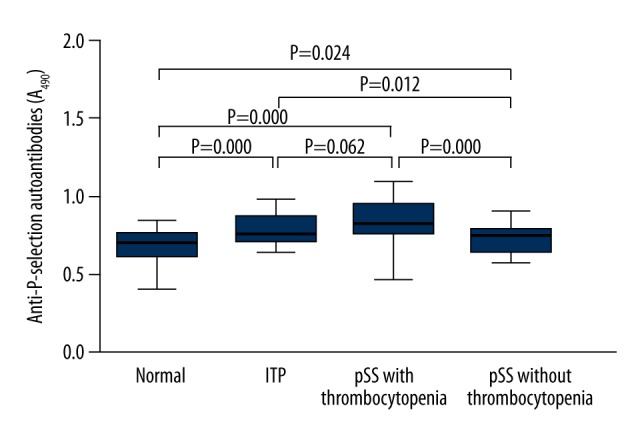
Plasma P-selectin autoantibodies (A490) in ITP patients, pSS patients with/without thrombocytopenia and healthy controls. Data are expressed as mean ±SD. The plasma P-selectin autoantibodies in ITP patients, pSS patients with thrombocytopenia and pSS patients without thrombocytopenia was significantly higher than in healthy controls (P=0.000, P=0.000 and P=0.024, respectively), but there was no significant difference in the P-selectin autoantibodies between ITP patients and pSS patients with thrombocytopenia (P=0.062).
Proportion of P-selectin autoantibodies-positive patients
The proportion of P-selectin autoantibodies-positive patients was also calculated (Figure 3). Of all the patients, 20.6% (21/102) were positive for P-selectin autoantibodies. The proportion of P-selectin autoantibodies-positive patients was 15.63% (5/32), 40.63% (13/32), and 7.89% (3/38) in ITP patients, pSS patients with thrombocytopenia, and pSS patients without thrombocytopenia, respectively. The proportion of P-selectin autoantibodies-positive patients in ITP patients and pSS patients with thrombocytopenia was markedly higher than that in healthy controls (P=0.015 and P=0.000, respectively), but no significant difference was observed between healthy controls and pSS patients without thrombocytopenia (P=0.090), and the positive rate in pSS patients with thrombocytopenia was also comparable to that in ITP patients (P=0.05). The anti-P-selectin autoantibodies-positive rate in ITP patients was significantly higher than that in pSS patients without thrombocytopenia (P=0.001).
Figure 3.
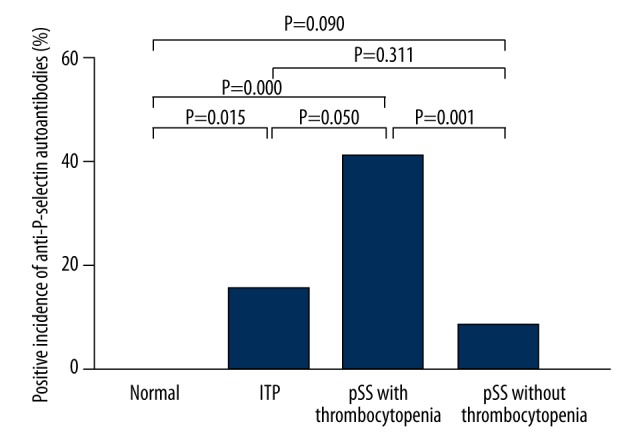
Proportion of P-selectin autoantibodies-positive patients. The proportion of P-selectin autoantibodies-positive patients in ITP patients, pSS patients with thrombocytopenia and pSS patients without thrombocytopenia was 15.63% (5/32), 40.63% (13/32) and 7.89% (3/38), respectively. There was significantly higher positive rate in ITP patients and pSS patients with thrombocytopenia than in healthy controls (P=0.015 and P=0.000, respectively), but no difference was observed between healthy controls and pSS patients without thrombocytopenia (P=0.090).
Relationship between P-selectin autoantibodies and platelet count
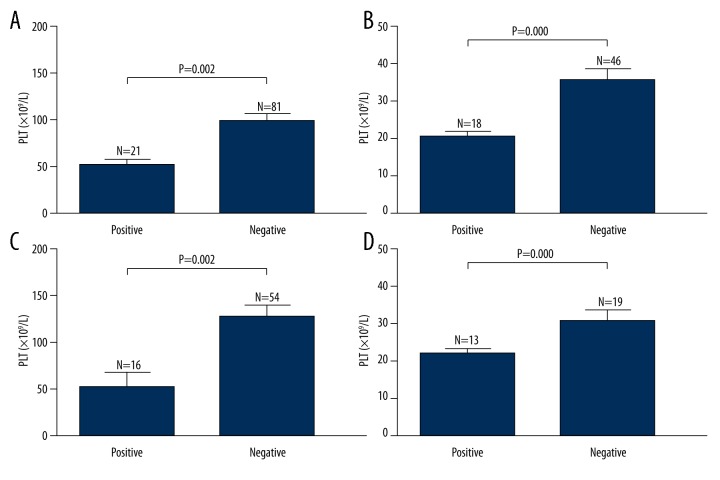
The relationship between P-selectin autoantibodies and platelet count was further evaluated in ITP patients and pSS patients with/without thrombocytopenia (Figure 4). There was a lower platelet count in P-selectin autoantibodies-positive patients (44.4±10.7×109/L) than in P-selectin autoantibodies-negative patients (98.6±80.9×109/L) (P=0.002) (Figure 4A). In ITP patients and pSS patients with thrombocytopenia, the platelet count in P-selectin autoantibodies-positive patients (20.8± 6.1×109/L) was lower than in anti-P-selectin autoantibodies-negative patients (35.9± 19.5×109/L) (P=0.000) (Figure 4B). There was a lower platelet count in P-selectin autoantibodies-positive pSS patients (53.1±67.5×109/L) than in P-selectin autoantibodies-negative pSS patients (128.4±83.1×109/L) (P=0.002) (Figure 4C), while the platelet count in P-selectin autoantibodies-positive patients (22.3±5.2×109/L) was markedly lower than in P-selectin autoantibodies-negative patients (31.1±12.7×109/L) (P=0.012) among pSS patients with thrombocytopenia (Figure 4D).
Figure 4.
Platelet count in anti-P-selectin autoantibodies-positive/negative patients with ITP and pSS. (A) Platelet count in P-selectin autoantibodies-positive/negative patients with ITP and pSS. There was a lower platelet count in P-selectin autoantibodies-positive patients than in P-selectin autoantibodies-negative patients (P=0.002). (B) Platelet count in P-selectin autoantibodies-positive/negative ITP patients and pSS patients with thrombocytopenia. There was a lower platelet count in P-selectin autoantibodies-positive patients than in P-selectin autoantibodies-negative patients (P=0.000) among ITP patients and pSS patients with thrombocytopenia. (C) Platelet count in P-selectin autoantibodies-positive/negative pSS patients. There was a lower platelet count in P-selectin autoantibodies-positive pSS patients than in P-selectin autoantibodies-negative pSS patients (P=0.002). (D) Platelet count in P-selectin autoantibodies-positive/negative pSS patients with thrombocytopenia. There was a lower platelet count in P-selectin autoantibodies-positive patients than in anti-P-selectin autoantibodies-negative patients (P=0.012).
Discussion
The present study demonstrated that the plasma P-selectin autoantibodies significantly increased in pSS patients with thrombocytopenia as compared to ITP patients, pSS patients without thrombocytopenia, and healthy controls. Additionally, the proportion of P-selectin autoantibodies-positive patients was markedly higher in pSS patients with thrombocytopenia, and being positive for P-selectin autoantibodies was associated with a low platelet count, indicating that P-selectin autoantibodies may be involved in the destruction of platelets and the pathogenesis of thrombocytopenia in pSS.
pSS is a systemic autoimmune disease characterized by inflammation that can impair multiple organs, and the impairment of the hematopoietic system is common. Both T cells [25] and B cells [7] play important roles in the development of pSS. B-cell hyperactivity is a major characteristic of pSS and is predominantly characterized by the production of a series of autoantibodies against various autoantigens, such as Ro52/TRIM21, Ro60/TROVE2, La/SSB, U1RNP, RF, cryoglobulins, centromere (ACA), mitochondria (AMA), smooth muscle, cyclic citrullinated peptides (anti-CCP), and others [26]. ANA is found in 59–85% of pSS patients [27], but is not specific for pSS. Ro/SSA antibody and La/SSB antibody are the major autoantibodies in pSS and can be used in the diagnosis of pSS [23]. Ro/SSA antibodies represent 2 distinct entities of autoantibodies: Ro52/TRIM21 antibodies and Ro60/TROVE2 antibodies. Ro52/TRIM21 antibodies are the most common specific autoantibody, and are detectable in 66.7% of pSS patients [28]. Ro60/TROVE2 antibodies and La/SSB antibodies are found in 52.1% and 49% of pSS patients, respectively [29]. RF is another family of autoantibodies and is present in 36–74% of pSS patients [6,30,31].
As a member of the selectin family, P-selectin is stored in the granules of platelets and the Weibel-Palade bodies of endothelial cells [32,33]. When the platelets and endothelial cells are activated, P-selectin is expressed on the cell surface and released into the plasma as a soluble form [34]. Increased soluble P-selectin is related to thrombotic diseases [20] and ITP is also an autoimmune disorder; several autoantibodies against GP such as GPIb, GP IX, GPIIb/IIIa, and P-selectin have been identified in ITP patients [16]. P-selectin autoantibodies may lead to platelet destruction and impair the platelet function. Although there is a role of P-selectin autoantibodies in the pathogenesis of ITP, the association between P-selectin autoantibodies and pSS with thrombocytopenia remains unknown.
Our results demonstrated that the plasma P-selectin autoantibodies were significantly higher in pSS patients with thrombocytopenia than in ITP patients. P-selectin autoantibodies-positive rate in pSS patients with thrombocytopenia was also markedly higher than in ITP patients. Moreover, there was a lower platelet count in P-selectin autoantibodies-positive patients. There were both endothelial injury and platelet activation in pSS patients, which may lead to platelet destruction. Therefore, the increase in P-selectin autoantibodies in pSS patients with thrombocytopenia was more obvious than in ITP patients, and the platelet count in P-selectin autoantibodies-positive pSS patients with thrombocytopenia was lower than in ITP patients. Our results suggest that the increased plasma P-selectin autoantibodies may contribute to the platelet destruction in pSS patients and finally result in thrombocytopenia. To the best of our knowledge, this is the first report on the role of P-selectin autoantibodies in the thrombocytopenia of pSS patients.
There were several limitations in this study. First, this was a retrospective study and patients were not followed up. Second, only patients older than 18 years were enrolled; therefore, the results might not be generalizable to children. Third, the sample size was small and patients were enrolled from a single center.
Conclusions
We for the first time report the role of P-selectin autoantibodies in the thrombocytopenia of pSS patients. P-selectin autoantibodies may lead to platelet destruction and endothelial injury and play a role in the pathogenesis of thrombocytopenia in pSS. Further prospective studies are still needed to understand the potential mechanism underlying the role of P-selectin autoantibodies in thrombocytopenia of pSS patients.
Footnotes
References
- 1.Fox RI. Sjögren’s syndrome. Lancet. 2005;366:321–31. doi: 10.1016/S0140-6736(05)66990-5. [DOI] [PubMed] [Google Scholar]
- 2.Ataoglu EH, Demir B, Tuna M, et al. Sjögren syndrome presenting with hypopotassemic periodic paralysis due to renal tubular acidosis. Am J Case Rep. 2012;13:187–90. doi: 10.12659/AJCR.883326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maldini C, Seror R, Fain O, et al. Epidemiology of primary Sjögren’s syndrome in a French multiracial/multiethnic area. Arthritis Care Res (Hoboken) 2014;66:454–63. doi: 10.1002/acr.22115. [DOI] [PubMed] [Google Scholar]
- 4.Kittridge A, Routhouska SB, Korman NJ. Dermatologic manifestations of Sjögren syndrome. J Cutan Med Surg. 2011;15:8–14. doi: 10.2310/7750.2010.09033. [DOI] [PubMed] [Google Scholar]
- 5.Ramos-Casals M, Font J, Garcia-Carrasco M, et al. Primary Sjögren syndrome: hematologic patterns of disease expression. Medicine (Baltimore) 2002;81:281–92. doi: 10.1097/00005792-200207000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Ramos-Casals M, Solans R, Rosas J, et al. Primary Sjögren syndrome in Spain: clinical and immunologic expression in 1010 patients. Medicine (Baltimore) 2008;87:210–19. doi: 10.1097/MD.0b013e318181e6af. [DOI] [PubMed] [Google Scholar]
- 7.Cornec D, Devauchelle-Pensec V, Tobon GJ, et al. B cells in Sjögren’s syndrome: from pathophysiology to diagnosis and treatment. J Autoimmun. 2012;39:161–67. doi: 10.1016/j.jaut.2012.05.014. [DOI] [PubMed] [Google Scholar]
- 8.Bournia VK, Vlachoyiannopoulos PG. Subgroups of Sjögren syndrome patients according to serological profiles. J Autoimmun. 2012;39:15–26. doi: 10.1016/j.jaut.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 9.Tzioufas AG, Kapsogeorgou EK, Moutsopoulos HM. Pathogenesis of Sjögren’s syndrome: what we know and what we should learn. J Autoimmun. 2012;39:4–8. doi: 10.1016/j.jaut.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 10.Jonsson R, Theander E, Sjostrom B, et al. Autoantibodies present before symptom onset in primary Sjögren syndrome. JAMA. 2013;310:1854–55. doi: 10.1001/jama.2013.278448. [DOI] [PubMed] [Google Scholar]
- 11.Manganelli P, Fietta P, Quaini F. Hematologic manifestations of primary Sjögren’s syndrome. Clin Exp Rheumatol. 2006;24:438–48. [PubMed] [Google Scholar]
- 12.Aoki A, Ohno S, Ueda A, et al. Hematological abnormalities of primary Sjögren’s syndrome. Nihon Rinsho Meneki Gakkai Kaishi. 2000;23:124–28. doi: 10.2177/jsci.23.124. [in Japanese] [DOI] [PubMed] [Google Scholar]
- 13.Harris EN, Asherson RA, Gharavi AE, et al. Thrombocytopenia in SLE and related autoimmune disorders: association with anticardiolipin antibody. Br J Haematol. 1985;59:227–30. doi: 10.1111/j.1365-2141.1985.tb02988.x. [DOI] [PubMed] [Google Scholar]
- 14.Stasi R, Newland AC. ITP: a historical perspective. Br J Haematol. 2011;153:437–50. doi: 10.1111/j.1365-2141.2010.08562.x. [DOI] [PubMed] [Google Scholar]
- 15.Cines DB, Blanchette VS. Immune thrombocytopenic purpura. N Engl J Med. 2002;346:995–1008. doi: 10.1056/NEJMra010501. [DOI] [PubMed] [Google Scholar]
- 16.He Y, Zhao YX, Zhu MQ, et al. Detection of autoantibodies against platelet glycoproteins in patients with immune thrombocytopenic purpura by flow cytometric immunobead array. Clin Chim Acta. 2013;415:176–80. doi: 10.1016/j.cca.2012.10.035. [DOI] [PubMed] [Google Scholar]
- 17.Sellam J, Proulle V, Jungel A, et al. Increased levels of circulating microparticles in primary Sjögren’s syndrome, systemic lupus erythematosus and rheumatoid arthritis and relation with disease activity. Arthritis Res Ther. 2009;11:R156. doi: 10.1186/ar2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blann AD, Nadar SK, Lip GY. The adhesion molecule P-selectin and cardiovascular disease. Eur Heart J. 2003;24:2166–79. doi: 10.1016/j.ehj.2003.08.021. [DOI] [PubMed] [Google Scholar]
- 19.Andre P, Hartwell D, Hrachovinova I, et al. Pro-coagulant state resulting from high levels of soluble P-selectin in blood. Proc Natl Acad Sci USA. 2000;97:13835–40. doi: 10.1073/pnas.250475997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Myers DD, Hawley AE, Farris DM, et al. P-selectin and leukocyte microparticles are associated with venous thrombogenesis. J Vasc Surg. 2003;38:1075–89. doi: 10.1016/s0741-5214(03)01033-4. [DOI] [PubMed] [Google Scholar]
- 21.Cambien B, Wagner DD. A new role in hemostasis for the adhesion receptor P-selectin. Trends Mol Med. 2004;10:179–86. doi: 10.1016/j.molmed.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 22.Polgar J, Matuskova J, Wagner DD. The P-selectin, tissue factor, coagulation triad. J Thromb Haemost. 2005;3:1590–96. doi: 10.1111/j.1538-7836.2005.01373.x. [DOI] [PubMed] [Google Scholar]
- 23.Vitali C, Bombardieri S, Jonsson R, et al. Classification criteria for Sjögren’s syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis. 2002;61:554–58. doi: 10.1136/ard.61.6.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rodeghiero F, Stasi R, Gernsheimer T, et al. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an international working group. Blood. 2009;113:2386–93. doi: 10.1182/blood-2008-07-162503. [DOI] [PubMed] [Google Scholar]
- 25.Singh N, Cohen PL. The T cell in Sjögren’s syndrome: force majeure, not spectateur. J Autoimmun. 2012;39:229–33. doi: 10.1016/j.jaut.2012.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kyriakidis NC, Kapsogeorgou EK, Tzioufas AG. A comprehensive review of autoantibodies in primary Sjögren’s syndrome: clinical phenotypes and regulatory mechanisms. J Autoimmun. 2014;51:67–74. doi: 10.1016/j.jaut.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 27.Nardi N, Brito-Zeron P, Ramos-Casals M, et al. Circulating auto-antibodies against nuclear and non-nuclear antigens in primary Sjögren’s syndrome: prevalence and clinical significance in 335 patients. Clin Rheumatol. 2006;25:341–46. doi: 10.1007/s10067-005-0059-3. [DOI] [PubMed] [Google Scholar]
- 28.Oke V, Wahren-Herlenius M. The immunobiology of Ro52 (TRIM21) in autoimmunity: a critical review. J Autoimmun. 2012;39:77–82. doi: 10.1016/j.jaut.2012.01.014. [DOI] [PubMed] [Google Scholar]
- 29.Routsias JG, Tzioufas AG. Sjögren’s syndrome – study of autoantigens and autoantibodies. Clin Rev Allergy Immunol. 2007;32:238–51. doi: 10.1007/s12016-007-8003-8. [DOI] [PubMed] [Google Scholar]
- 30.Fauchais AL, Martel C, Gondran G, et al. Immunological profile in primary Sjögren syndrome: clinical significance, prognosis and long-term evolution to other auto-immune disease. Autoimmun Rev. 2010;9:595–99. doi: 10.1016/j.autrev.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 31.Martel C, Gondran G, Launay D, et al. Active immunological profile is associated with systemic Sjögren’s syndrome. J Clin Immunol. 2011;31:840–47. doi: 10.1007/s10875-011-9553-3. [DOI] [PubMed] [Google Scholar]
- 32.Stenberg PE, McEver RP, Shuman MA, et al. A platelet alpha-granule membrane protein (GMP-140) is expressed on the plasma membrane after activation. J Cell Biol. 1985;101:880–86. doi: 10.1083/jcb.101.3.880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bonfanti R, Furie BC, Furie B, Wagner DD. PADGEM (GMP140) is a component of Weibel-Palade bodies of human endothelial cells. Blood. 1989;73:1109–12. [PubMed] [Google Scholar]
- 34.Dunlop LC, Skinner MP, Bendall LJ, et al. Characterization of GMP-140 (P-selectin) as a circulating plasma protein. J Exp Med. 1992;175:1147–50. doi: 10.1084/jem.175.4.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]