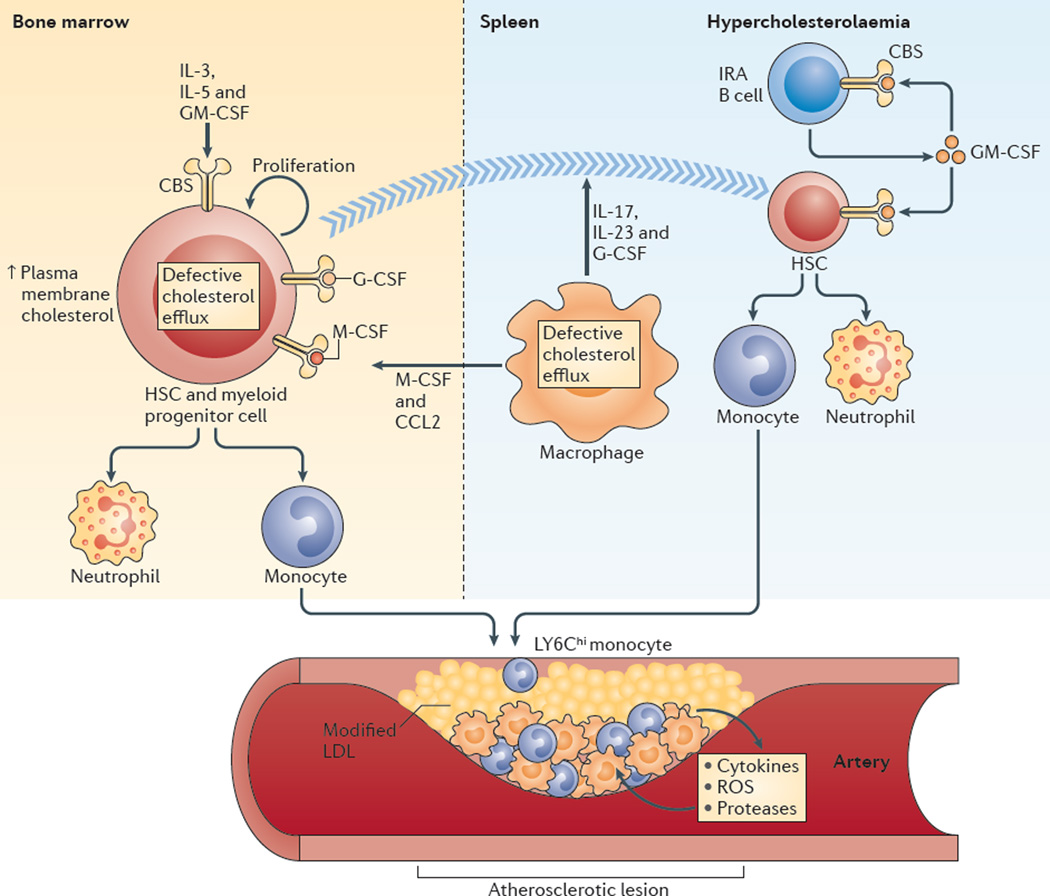
Figure 4. Hypercholesterolaemia and defective cholesterol efflux promote myelopoiesis and atherosclerosis.
In the bone marrow, increased plasma membrane cholesterol content in haematopoietic stem cells (HSCs) and myeloid progenitor cells as a result of defective cholesterol efflux promotes increased cell surface levels of the common β-subunit (CBS) of the interleukin-3 (IL-3), IL-5 and granulocyte–macrophage colony-stimulating factor (GM-CSF) receptors and increased proliferation in response to these growth factors. Extramedullary haematopoiesis can also occur after HSCs progressively relocate from the bone marrow to the splenic red pulp. Efferocytosis in the setting of defective cholesterol efflux in macrophages fails to suppress the production of IL-17, IL-23 and granulocyte colony-stimulating factor (G-CSF), and these cytokines can promote HSC relocation to the spleen. In the spleen, the number of GM-CSF-producing innate response activator B cells (IRA B cells) increases in mice with hypercholesterolaemia, which causes increased production of monocytes and neutrophils from HSCs. In addition, defective cholesterol efflux in splenic macrophages can promote the development of monocytes from HSCs and myeloid progenitor cells in the bone marrow via macrophage colony-stimulating factor (M-CSF) and CC-chemokine ligand 2 (CCL2). Monocytes, especially LY6Chi monocytes produced in the bone marrow and the spleen, enter the bloodstream and accumulate in atherosclerotic lesions. LDL, low-density lipoprotein; ROS, reactive oxygen species.