Figure 3.
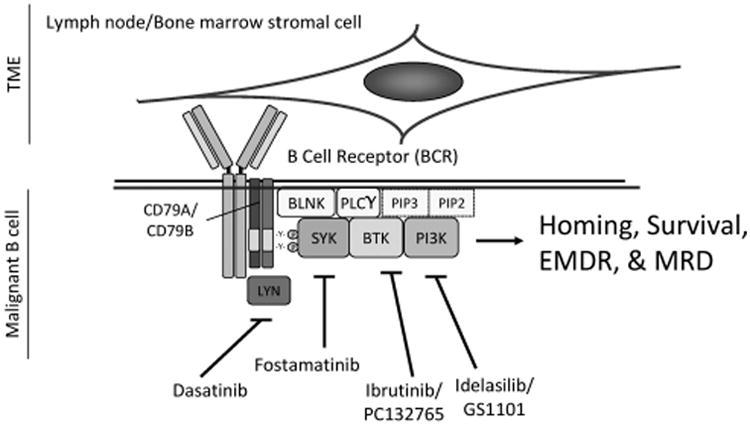
Targeting BCR signaling attenuates homing, survival, EMDR and MRD of malignant B cells. The BCR is a transmembrane protein located on the outer surface of B cells. It is a heterodimer composed of heavy-chain and light-chain Igs, CD79A and CD79B. Upon ligation by antigen, the immune receptor tyrosine activation motif domains of CD79A and CD79B are phosphorylated by the src-family tyrosine kinase, LYN, and spleen tyrosine kinase, SYK. BCR phosphorylation facilitates recruitment of additional kinases and adapter proteins including Bruton's tyrosine kinase (BTK), B-cell linker (BLNK) and other adapter proteins forming a large protein multimer or signalome. CD79A/CD79B, LYN, SYK, PLCγ2, PI3Ks and BTK comprise the core intrinsic signaling determinants of BCR. The data discussed in this review indicate that the BCR is a central hub for the integration between the extrinsic B-cell microenvironment and the intrinsic signaling pathways, thereby playing a central role in malignant B-cell homing, survival and EMDR. To this end, targeting the BCR pathway will attenuate growth and survival signals emanating from both B-cell intrinsic abnormalities and from the TME. BCR-targeting strategies using SYK inhibitor fosamatinib, the BTK inhibitor ibrutinib and the PI3Kδ inhibitor GS1101/idelalisib revealed that patients with malignant B-cell disorders are particularly sensitive to inhibitors of BCR-associated kinases.
