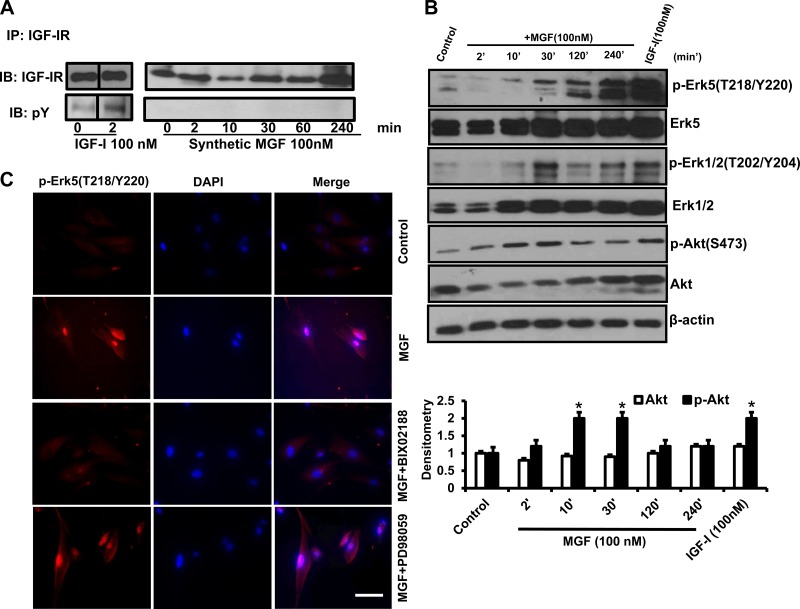
Fig. 3.
Synthetic MGF activates Erk-5 and induces Erk-5 nuclear translocation. A: rhIGF-I but not synthetic MGF induced IGF-I receptor phosphorylation. IGF-IR phosphorylation was measured in phosphotyrosine immunoblots of IGF-IR immunoprecipitates of human intestinal smooth muscle cells stimulated with synthetic MGF (100 nM) or rhIGF-I (100 nM). B: representative immunoblots showing that synthetic MGF (100 nM) elicited time-dependent phosphorylation of Erk5(T218/Y220) and Erk1/2(T202/Y204) but only modest transient phosphorylation of Akt(S473) in normal human intestinal muscle cells. In contrast, in addition to Erk5(T218/Y220) and Erk1/2(T202/Y204), rhIGF-I (100 nM, 2 min) also elicits Akt(S473) phosphorylation in primary cultures of normal human intestinal muscle. C: synthetic MGF (100 nM) elicited prompt, within 10 min, Erk-5(T218/Y220) phosphorylation and nuclear translocation that was inhibited by the selective Erk-5 inhibitor BIX02188 (30 μM). Cell nuclei were counterstained with DAPI. *P < 0.05 vs. control.