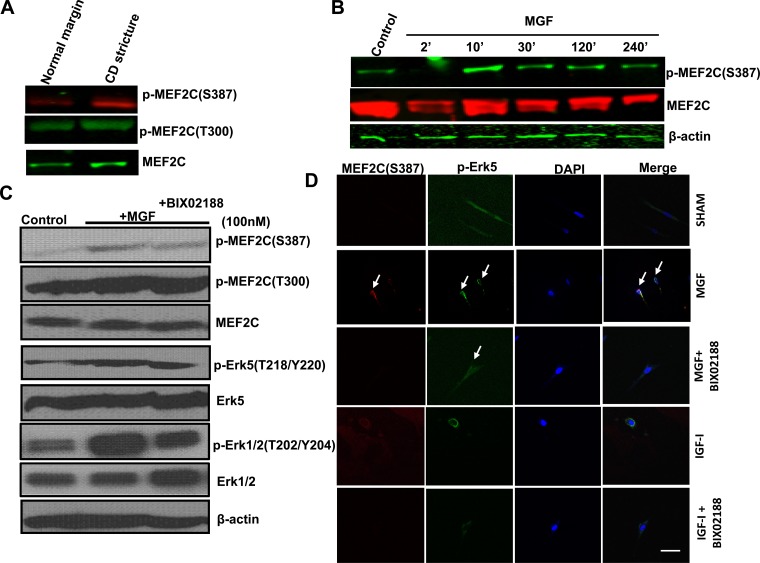
Fig. 4.
MEF2C activity is increased in muscle cells of stricturing Crohn's disease and increased in normal muscle cells by synthetic MGF. A: phosphorylation of MEF2C(S387) but not MEF2C(T300) is increased in muscle cells isolated from strictured intestine compared with normal resection margin in the same patient with stricturing Crohn's disease. B: synthetic MGF (100 nM) elicits time-dependent MEF2C(S387) phosphorylation in normal human intestinal muscle cells. MEF2C phosphorylation was measured by Western blot analysis. C: synthetic MGF (100 nM)-induced phosphorylation of MEF2C(S387) and Erk5(T218/Y220) was decreased in the presence of the selective Erk5 inhibitor BIX02188 in muscle cells isolated from normal resection margin. D: synthetic MGF-induced phosphorylation of Erk5(T218/Y220), colocalization with MEF2C and (indicated by white arrow) that was lost in the presence of the selective Erk-5 inhibitor BIX02188. In contrast, rhIGF-I (100 nM) had no effect on colocalization or nuclear translocation of Erk5 and MEF2C.