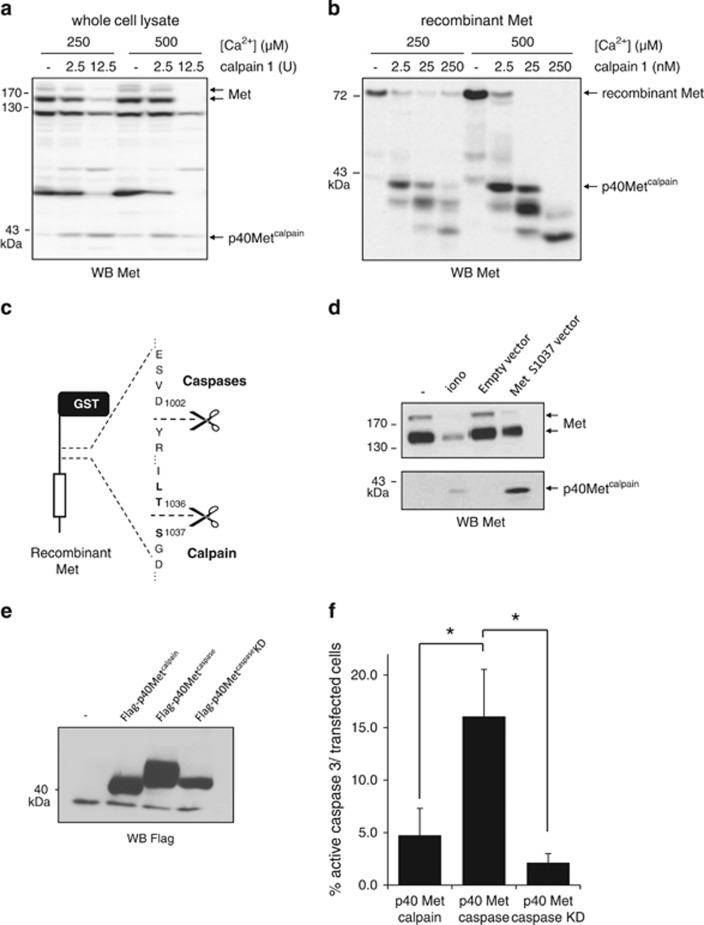
Figure 4.
Reconstitution of p40Metcalpain and evaluation of its apoptotic properties. (a) Equal amounts of extract obtained by lysing MCF-10A cells in LR buffer or (b) 5 ng purified recombinant intracellular Met domain were incubated with the indicated concentration of CaCl2 and purified calpain 1 for 30 min. (c) Schematic representation of the caspase and calpain cleavage sites in the juxtamembrane domain of Met. (d) MCF-10 A cells were grown for 24 h and either serum starved and treated for 1 h with 1 μM ionomycin (iono) or transfected with a vector expressing the Met S1037 variant. (a, b and d) Cell lysates were analyzed by western blotting with an antibody directed against the kinase domain of human Met. Arrows indicate the positions of Met, recombinant Met, and p40Metcalpain. (e) HEK cells were transfected with empty vector or a vector expressing Flag-tagged versions of p40Metcaspase, p40Metcaspase K1108A (kinase dead, KD), or p40Metcalpain. Twenty-four hours later, the cells were lysed and lysates were analyzed by western blotting with an antibody directed against Flag. (f) MCF-10A cells were cultured on glass coverslips and transfected with the indicated Flag-p40Met. Twenty-four hours later, the cells were fixed and stained with antibodies against Flag and active caspase 3. Flag-positive cells (expressing p40 Met) and displaying active caspase 3 staining were counted. About 1000 flag-positive cells were counted per well and percentage was calculated (n=3; ±S.D.). The P-value of Student's t-test is shown (*P<0.05)