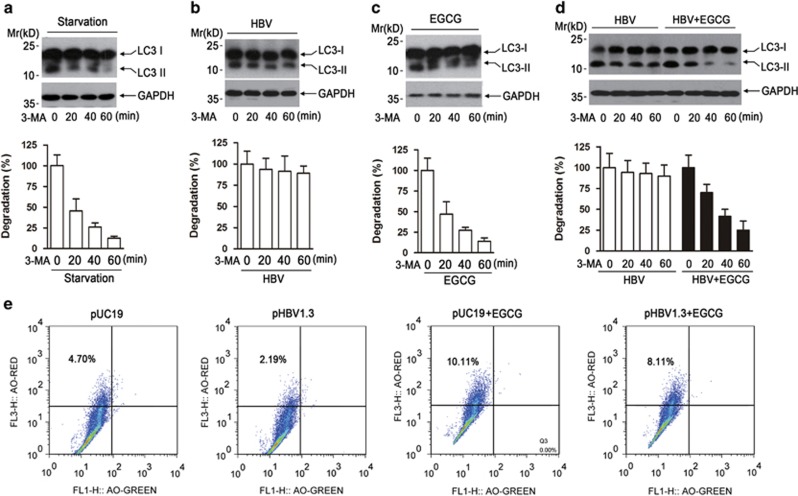
Figure 6.
EGCG opposes HBV-induced incomplete autophagy by increasing lysosomal acidification. (a) The effect of starvation on the degradation of formed autophagesome. HepG2 cells were cultured in Earle's Balanced Salt Solution medium for 3 h, and the pI3KC3 inhibitor 3-MA (10 mM) was added to stop the formation of new autophagosome. At the indicated time points (0, 20, 40, 60 min) after 3-MA addition, western blotting was used to determine the level of LC3-II. The expression of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as a loading control. Lower panel, the immunoblots from three independent experiments were scanned and subjected to densitometric analysis. The density value from starved cells without 3-MA treatment was set as 100%, bars represent means±S.E.M (n=3). (b) The effect of HBV transfection on the degradation of formed autophagesome. HepG2 cells were transfected with pHBV1.3 for 48 h. The degradation of formed autophagosome was examined as in panel (a). (c) The effect of EGCG treatment on the degradation of formed autophagesome. HepG2 cells were treated with EGCG for 24 h, and the degradation of formed autophagosome was examined as in panel (a). (d) The effect of HBV transfection on the degradation of formed autophagesome in the presence of EGCG. HepG2 cells were transfected with pHBV1.3. Forty-eight hours posttransfection, cells were then treated or untreated with 50 μM of EGCG for another 24 h. The degradation of formed autophagesome was determined as in panel (a). (e) The effect of HBV on the lysosomal acidification in the presence or absence of EGCG. HepG2 cells were transfected with pUC19 or pHBV1.3 for 48 h, followed by treatment with or without 50 μM of EGCG for 24 h. Cells were then stained with AO for 15 min and subjected to FACS analysis