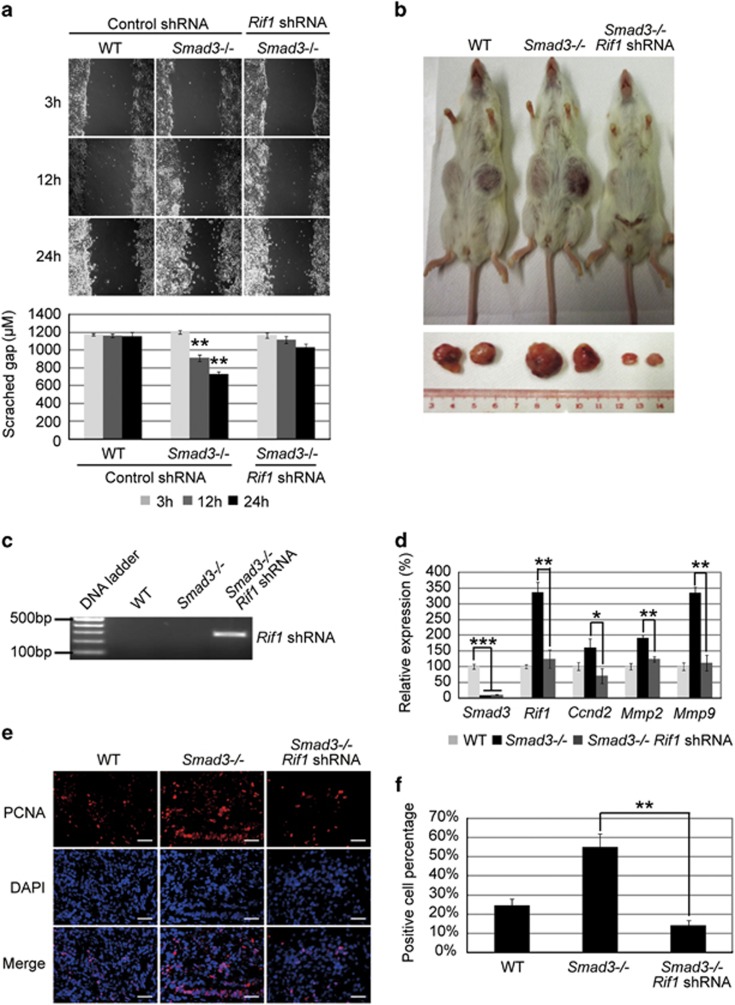
Figure 6.
Knockdown of Rif1 in Smad3−/− ESCs can attenuate cell migration. (a) Image (upper) and histogram (lower) of scratch wound healing of WT ESCs and control shRNA- and Rif1 shRNA-transduced Smad3−/− ESC-differentiated cells at 3, 12 and 24 h after scratch. The data are shown as the mean±S.D. (n=2). (b) Picture of SCID mice with tumors at 4 weeks after WT ESCs, Smad3−/− ESCs and Rif1 shRNA-transduced Smad3−/− ESCs were subcutaneously injected into SCID mice. (c) Genotyping of ES cell formed tumors to confirm Rif1 shRNA integration in Rif1 shRNA transduced Smad3−/− ES cells. (d) Quantitative real-time PCR to examine the mRNA levels of Smad3, Rif1, Ccnd2, Mmp2 and Mmp9 in tumors grown from WT ESCs, Smad3−/− ESCs and Rif1 shRNA-transduced Smad3−/− ESCs. Actin was analyzed as an internal control. The data are shown as the mean±S.D. (n=3). (e) Immunofluorescence staining with anti-PCNA antibody to examine PCNA expression in WT ESC, control Smad3−/− ESC and Rif1 shRNA-transduced Smad3−/− ESC-formed teratomas. The nuclei were stained with DAPI. Scale bar=200 μm. (f) Quantification of the PCNA-positive cell percentage compared with DAPI in WT ESC, control Smad3−/− ESC and Rif1 shRNA transduced Smad3−/− ESC-formed teratomas. Statistically significant differences, calculated through student's t-tests, are indicated (*P<0.05; **P<0.01; ***P<0.001)