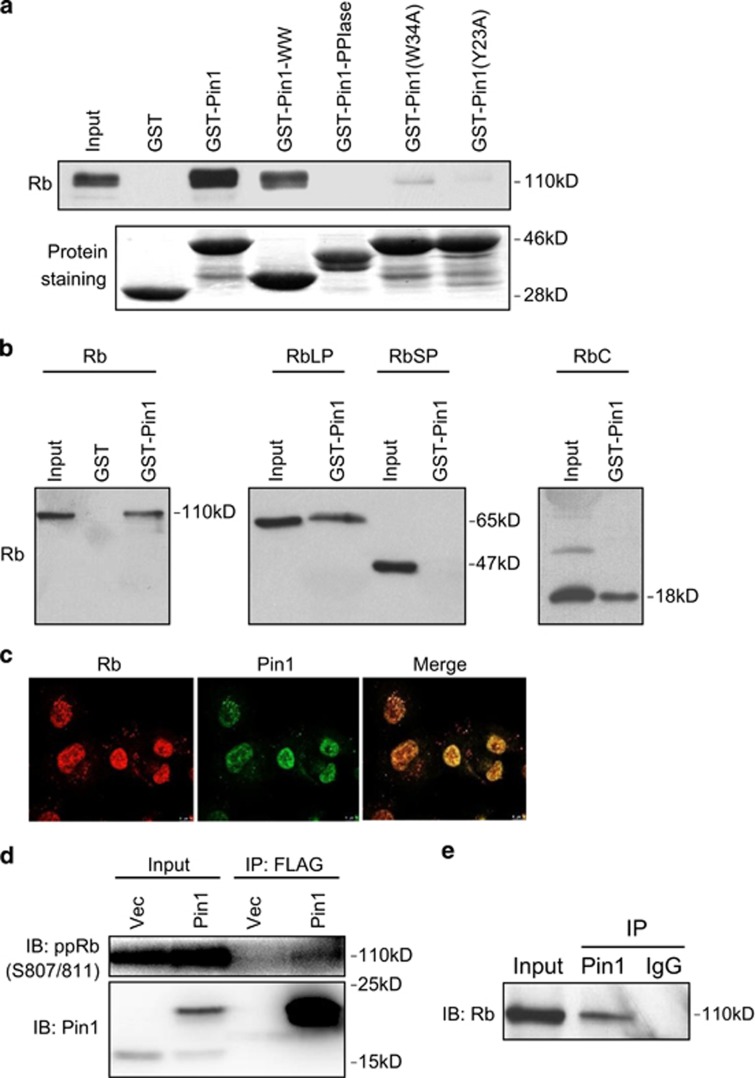
Figure 1.
The Pin1 WW domain directly binds to the hyperphosphorylated Rb C-pocket. (a) U2-OS cell lysates were incubated with full-length, truncated or mutant Pin1-GST fusion constructs and subsequently subjected to GST pull-down assay, as shown. Proteins were separated by SDS-PAGE and immunoblotted with an Rb-specific antibody (top panel). Comparable levels of input GST or GST fusion proteins are shown by Coomassie blue staining (lower panel). (b) U2-OS cells were transiently transfected with full-length Rb, RbLP, Rb small pocket (RbSP) or Rb C-pocket (RbC). Total protein (500 μg) was subjected to GST pull-down assay using recombinant GST-Pin1 or GST as a control and then analyzed by western blotting for Rb binding. Total protein (10 μg) from each cell lysate was directly loaded as input controls. (c) H1299 cells were subjected to immunofluorescence, as shown. (d) Cell lysates from H1299 cells stably expressing FLAG-Pin1 or pLVX vector were subjected to immunoprecipitation with anti-FLAG M2 Affinity Gel, and immunoblotted for phosphorylated Rb (pRb) at Ser807/811 (S807/811) or Pin1, as shown. (e) U2-OS cell lysates were subjected to immunoprecipitation with a Pin1-specific antibody or a control IgG, and immunoblotted for Rb, as shown