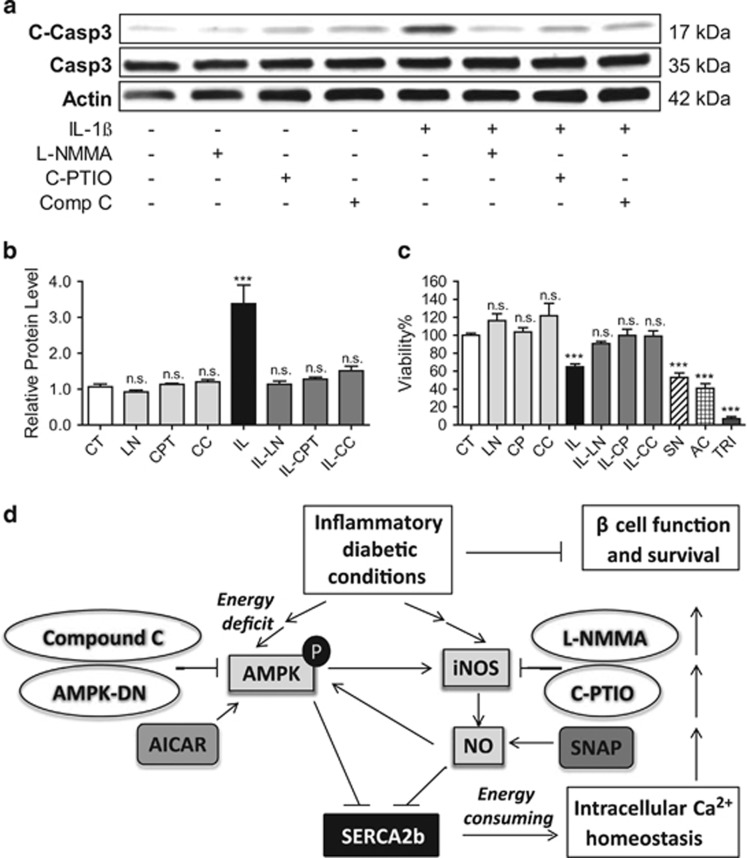
Figure 8.
Inhibition of iNOS and AMPK protect INS-1 cells from IL-1β-induced apoptosis. INS-1 cells were treated with dimethyl sulfoxide (DMSO) (CT) or 5 ng/ml IL-1β (IL) combined with or without 0.5 mM of the NOS inhibitor l-NMMA (LN) or 100 μM of the NO scavenger C-PTIO (CPT) for 24 h (a–c). Total protein was isolated, and immunoblot was performed using antibodies against cleaved caspase-3, total caspase-3 and actin. (b) Ratios of the relative expression of cleaved caspase-3 to total caspase-3 are shown graphically. (c) Cell viability assays were performed as described in the Materials and Methods section; 10% Triton treatment for 10 min was used as positive control for cell death. Indicated comparisons are significantly different (***P<0.001). (d) Our overall model suggests that under proinflammatory and diabetic conditions, activation of NO-mediated signaling in the pancreatic β-cell leads to an alteration in cellular energy status and activation of the master cellular energy sensor AMPK. The convergence of NO and AMPK signaling leads to a restraint of SERCA2b activity and altered SERCA2b protein stability, and the consequence of reduced SERCA2b expression and activity is dysregulation of intracellular and ER Ca2+ homeostasis that ultimately leads to a loss of β-cell function and survival. Whereas NO may initiate the activation of AMPK, our data also suggest that AMPK amplifies the inflammatory response of the pancreatic β-cell