Abstract
This study analyzed the relationship between electrophysiological responses to transcranial magnetic stimulation (TMS), finger tracking accuracy, and volume of neural substrate in children with congenital hemiparesis. Nineteen participants demonstrating an ipsilesional motor-evoked potential (MEP) were compared with eleven participants showing an absent ipsilesional MEP response. Comparisons of finger tracking accuracy from the affected and less affected hands and ipsilesional/contralesional (I/C) volume ratio for the primary motor cortex (M1) and posterior limb of internal capsule (PLIC) were done using two-sample t-tests. Participants showing an ipsilesional MEP response demonstrated superior tracking performance from the less affected hand (p = 0.016) and significantly higher I/C volume ratios for M1 (p= 0.028) and PLIC (p = 0.005) compared to participants without an ipsilesional MEP response. Group differences in finger tracking accuracy from the affected hand were not significant. These results highlight differentiating factors amongst children with congenital hemiparesis showing contrasting MEP responses: less affected hand performance and preserved M1 and PLIC volume. Along with MEP status, these factors pose important clinical implications in pediatric stroke rehabilitation. These findings may also reflect competitive developmental processes associated with the preservation of affected hand function at the expense of some function in the less affected hand.
Keywords: stroke, transcranial magnetic stimulation, pediatrics, corticospinal tract, motor-evoked potential, hemiparesis
1.Introduction
The safety of repetitive transcranial magnetic stimulation (rTMS) applications in adult stroke has been supported (Carey et al., 2008; Khedr et al., 2005; Liepert et al., 2007), and recent investigations of rTMS in children with congenital hemiparesis from stroke and periventricular leukomalacia have also demonstrated safety (Gillick et al., 2015; Kirton et al., 2008). Supporting the efficacy of rTMS in adult and pediatric stroke rehabilitation, however, is challenging. First, individual variability in responsiveness to non-invasive brain stimulation exists in both healthy individuals and in those with stroke, thus adding to the complexity of formulating accurate conclusions and refining stimulation parameters (Bradnam et al., 2012; Cheeran et al., 2008; Maeda et al., 2000; Seniów et al., 2012). Second, stringent enrollment criteria aimed at achieving optimal homogeneity often yield small sample sizes that compromise statistical power and generalizability of findings to larger stroke populations. In particular, studies employing rTMS interventions and/or TMS-based outcome measures frequently require a resting oran active motor-evoked potential (MEP) from the ipsilesional primary motor cortex area (M1). The MEP is the muscle response measured with electromyography(EMG) following a TMS pulse to the motor region of the brain. Yet, MEPs from the ipsilesional hemisphere are often absent in individuals with stroke (Escudero et al., 1998; Kirton et al., 2010; Stinear et al., 2007), thus hindering patient recruitment.
An ipsilesional MEP depends on he integrity of the contralateral (crossed) corticospinal tract (CST) projections, with influence from the size and location of the lesion (Staudt et al., 2002). The MEP (or lack thereof) is also dependent on central nervous system maturation (Koh & Eyre, 1988; Nezu et al., 1997). During typical development, an activity-dependent withdrawal of ipsilateral (uncrossed) CST projections from the hemisphereensues (Eyre et al., 2001; Martin & Lee, 1999). When an early-onset of neurological injury such as congenital stroke occurs, these ipsilateral projections can persist and even enlarge, rather than withdraw, and eventually predominate over surviving contralateral projections from the ipsilesional hemisphere (Eyre et al., 2001, 2007; Martin & Lee, 1999). Such adaptation may be important to preserving a modicum of function in the affected hand amidst major unilateral brain damage. Previous investigations of MEPs in children and in young adults with congenital hemiplegia have confirmed the utility of MEPs in determining CST organization and resultant motor function (Carr et al., 1993; Holmström et al., 2010). Elicitable MEPs may therefore contribute valuable insight to pediatric stroke and subsequent rehabilitation.
In our previous study investigating a combined rTMS and constraint-induced movement therapy (CIMT) intervention in participants with congenital hemiparesis, 19 of the 36(53%) originally enrolled children screened onsite qualified to participate (Gillick et al., 2014). Of the 17 children excluded after enrollment, 11 children (65%) could not participate secondary to absent ipsilesional MEPs. The purpose of this current observational study was to analyze the relationship between elicitable MEPs, finger tracking accuracy, and volume of neural substrate in cortical and subcortical regions of interest on magnetic resonance images (MRI) in children with congenital hemiparesis. Finger tracking is a complex task encompassing multiple systems. Akin to MEP status, finger tracking may also elucidate valuable information related to CST maturation (Fietzek et al., 2000; Heinen et al., 1998). We hypothesize that children with an ipsilesional MEP will demonstrate significantly higher tracking performance from the affected hand and significantly higher amounts of PLIC and M1.
2.Methods
2.1 Participants
Thirty children (13 females) with a mean ± SD age of 10.4 ± 2.79 years enrolled in a previous rTMS/CIMT study (Gillick et al., 2014) were included in this investigation. Of these 30 children, 19 were included in the initial study and comprised the MEP group in this investigation. The remaining 11 children were excluded from the initial study due to the absence of a resting or an active ipsilesional MEP determined during initial TMS testing. These 11 participants formed the no-MEP group in this study. Inclusion criteria for all children were congenital hemiparesis due to ischemic stroke that occurred within one year of birth or periventricular leukomalacia validated by MRI, at least 10 degrees of active flexion and extension at the metacarpophalangeal (MCP) joint of the affected index finger, and aged between 8 and 17 years. Exclusion criteria for all children were seizure within the previous two years, neoplasm, metabolic disorders, hemorrhage, receptive aphasia, pregnancy, disorders of cellular migration and proliferation, indwelling metal or medical devices contraindicated with MRI and TMS, claustrophobia, and gross visual field cuts that would hinder task performance during functional MRI (fMRI). All children and their legally authorized representatives assented/consented to participation.
2.2 Neuroimaging
All participants completed an anatomical MRI to confirm stroke and to assess stroke characteristics. Anatomical images were acquired using a 3-Tesla magnet (Magnetom Trio, Siemens, Munich, Germany). Fluid attenuated inversion recovery images were collected and assessed by a pediatric neurologist to specify location, type of stroke and cortical and/or subcortical involvement. Additional detail regarding MRI protocol and data acquisition is stated in previous work (Gillick et al., 2014). Diffusion tensor imaging (DTI) was attempted, but excessive head motion in essentially all participants prevented group-level analysis. fMRI was attempted while participants performed a finger tracking task, as done previously in adults with stroke (Carey et al., 2002), but this also was not successful because of excessive head motion and mirroring activity between the two hands. These movement-related issues that hinder brain imaging validity do not diminish the value of the finger tracking tasks in measuring manual control inside the MRI scanner.
2.3 Defining Regions of Interest
A team of trained investigators blinded to MEP status used Brain Voyager (Brain Innovation B.V., Maastricht, Netherlands) software to manually define two regions of interest: 1) M1 and 2) posterior limb of the internal capsule (PLIC). M1 was defined as the grey matter encompassing the posterior half of the precentral gyrus (Dassonville et al., 2001) including the motor hand-knob region (Yousry et al., 1997) beginning with the appearance of the central sulcus and ending with the disappearance of the central sulcus. PLIC borders were defined as: 1) anterior: genu of internal capsule, 2) posterior: posterior edge of globus pallidus and onset of corona radiata, 3) medial: thalamus, 4) lateral: globuspallidus, 5) rostral: lentiform nucleus, and 6) caudal: anterior commissure (Schaechter et al., 2008). Investigators performed a volumetric analysis (voxel count) for ipsilesional and contralesional M1 and PLIC regions, and constructed volume symmetry ipsilesional/contralesional (I/C) ratios for each region. If the lesion intruded the ROI boundaries, only the area around but not within the lesion was included. We included the full volume of the ROI on the contralesional hemisphere.
2.4 Finger Tracking
For the fMRI performance task, participants wore custom-made electrogoniometers with potentiometers (ETI Systems Inc., Carlsbad, CA) positioned at the MCP joint of both index fingers while performing a finger tracking task separately with each hand. The tracking task occurred in the MRI scanner with children positioned in supine while performing active finger flexion and extension movements. A mirror attached to the head coil reflected a 21.5 cm high target waveform image to a 43 cm high by 49 cm wide screen approximately 30.5 cm from the participant. The task included 30-second alternating blocks of rest (7 blocks), affected finger tracking (3 blocks), and less affected finger tracking (3 blocks). Past work has shown improvement in tracking performance in healthy children after several hours of tracking training (Gauthier et al., 1988). Because participants in this study completed only one practice trial to ensure comprehension of visual cues, it is unlikely that training or learning effects occurred. However, to control for potential learning, participants were randomized to one of two block sequences with the first non-rest block beginning with either affected finger tracking or less affected finger tracking. Accuracy index (AI) scores of the tracking performance in each hand were computed using the following formula:
where P is the root-mean-square (RMS) difference between the sine wave and a midline separating the upper and lower phases of the sine wave and E is the RMS error between the subject's response and the target waveform (Carey et al., 2002). AI scores were normalized to the participant's available MCP joint range of motion to account for variation in excursion amongst participants. Maximum AI scores equal 100%. Negative scores are possible and indicate a poor tracking response as described previously (Carey et al., 2002, 1990). This finger tracking paradigm has been shown to be reliable and valid in people with stroke (Carey et al., 1998, 1990), and it has been used repeatedly to study both manual and ankle control in people with stroke in MRI scanners (Bhatt et al., 2007; Carey et al., 2002, 2007; Deng et al., 2012; Kimberley et al., 2004).
2.5 TMS Assessment of Ipsilesional Corticospinal Excitability
Participants sat in a reclining chair, donning earplugs for safety and a Lycra swimcap that enabled investigators to mark points for stimulation. Surface electrodes (Cadwell Laboratories, Kennewick, WA) were attached over the extensor digitorummuscle on the affected hand and connected to a Cadwell Sierra II EMG machine to monitor muscle activity. Single TMS pulses were delivered through a 70 mm figure-eight coil connected to a Magstim Rapid-200 stimulator (Magstim Company Limited, Dyfed, UK). The coil was positioned over the potential motor “hotspot” with the coil handle aligned in a 45-degree posterolateral direction from the sagittal line. Pulses were delivered at 0.1 Hz beginning at an intensity of 50% of maximum machine output. The intensity and position of the coil were adjusted until a resting motor threshold, defined as the lowest intensity that produced MEPs ≥ 50μV peak-to-peak amplitude in 3 of 5 trials, was found. If MEPs could not be found at rest, the participant was instructed to perform a mild voluntary contraction of their affected extensor digitorum muscle during TMS pulse delivery. This active motor threshold was defined as the intensity required toevoke an active MEP with a peak-to-peak amplitude ≥ 100 μV in 3 of 5 trials (Kirton et al., 2010). If neither resting nor active ipsilesional MEPs were found, the child was discontinued; however, the AI scores and I/C volume ratios of this “no-MEP group” were compared to those who did have an ipsilesional MEP (“MEP group”).
2.6 Statistical Analysis
Group (MEP vs. no-MEP) comparisons of primary outcome measures entailing AI scores from the affected and less affected hands and M1 and PLIC I/C volume ratios were performed using two-sample t-tests. Prior to the main group comparisons, potential group differences in age, sex, mirroring, and stroke hemisphere were completed using two-sample t-tests for continuous data and Fisher Exact Tests for categorical data. Associations between AI scores, I/C volume ratios, and Assisting Hand Assessment (AHA) logit-based scores (Krumlinde-Sundholm, 2012) were assessed using Pearson correlation coefficients. The AHA is a bimanual hand function test (Krumlinde- Sundholm et al., 2007). If a violation of normality occurred as indicated by the Shapiro-Wilk test, the corresponding non-parametric test (i.e. Mann Whitney U) was performed. A p-value ≤ 0.05 indicated significance. Consistent with reasoning provided by Pocock(1997), use of the Bonferroni correction for a smaller-sized exploratory study likely over-corrects for Type I error possibly hindering future study and advancement in the field. Therefore, we did not correct for multiple comparisons. Due to the exploratory nature of this study, formal power and sample size assessments were not done.
3.Results
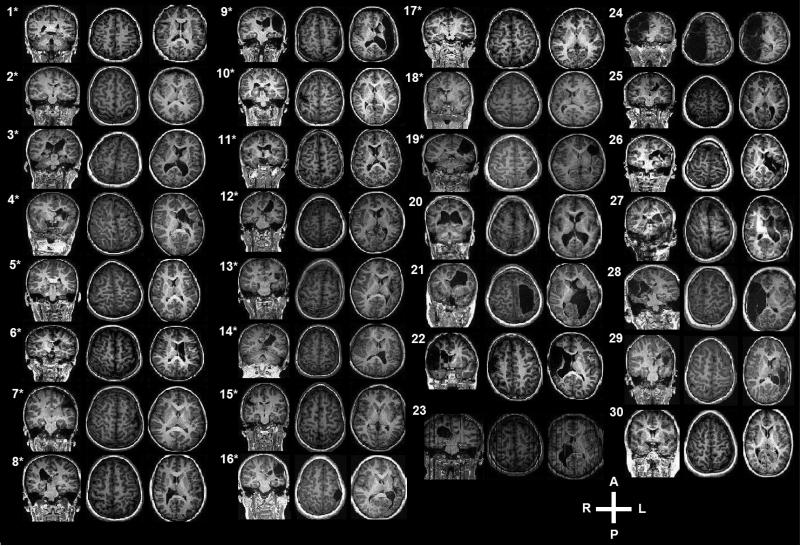
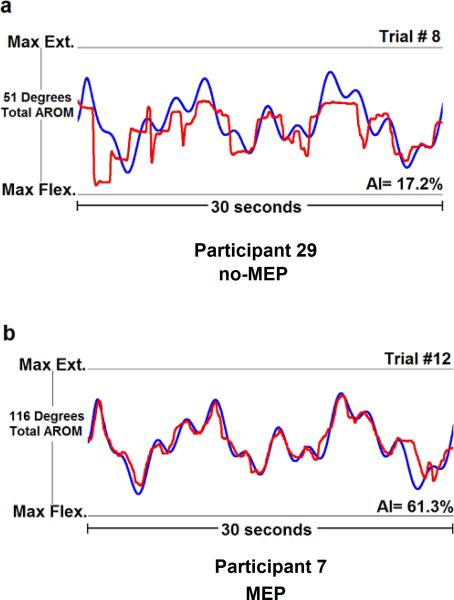
A summary of participant demographics and their corresponding anatomical MRIs are provided in Table 1 and Figure 1, respectively. Affected and less affected hand tracking scores were not available for one no-MEP participant. Affected hand tracking performance was not available for one MEP participant. The MEP group demonstrated significantly higher I/C volume ratios for M1 (p = 0.028) and PLIC (p = 0.005) and superior less affected hand finger tracking accuracy (p = 0.016) than the no-MEP group. Affected hand finger tracking accuracy, age, sex, mirroring, and stroke hemisphere did not significantly differ between groups (Table 2). Figure 2 illustrates representative examples of less affected hand finger tracking from participants in the MEP and no-MEP groups. No major adverse events occurred during TMS testing. Additional detail concerning safety is available (Gillick et al., 2015).
Table 1.
Participant Demographics
| Participant | Group | Threshold Type | Motor Threshold (% of Machine Output) | Sex | Age (y) | GMFCS | MACS | Stroke Hemisphere | Stroke Location |
|---|---|---|---|---|---|---|---|---|---|
| 1 | MEP | RMT | 39 | F | 15 | 1 | 1 | R | PVWM |
| 2 | MEP | RMT | 42 | F | 15 | 1 | 2 | L | LV, BG |
| 3 | MEP | RMT | 72 | M | 8 | 1 | 2 | L | T, P, posterior/anterior IC |
| 4 | MEP | RMT | 65 | M | 8 | 1 | 2 | L | T, P, BG, anterior IC, |
| 5 | MEP | RMT | 79 | M | 8 | 1 | 1 | L | MCA, CS, parietal/occipital/ frontal WM |
| 6 | MEP | AMT | 79 | M | 8 | 1 | 2 | L | T, P, BG, anterior IC |
| 7 | MEP | AMT | 74 | M | 8 | 1 | 2 | L | PFL |
| 8 | MEP | AMT | 66 | M | 12 | 1 | 2 | R | CS, BG, T |
| 9 | MEP | RMT | 69 | F | 12 | 1 | 2 | L | BG, MCA, PFL T |
| 10 | MEP | AMT | 78 | F | 8 | 1 | 2 | R | CR, PFL |
| 11 | MEP | AMT | 60 | M | 9 | 1 | 2 | L | IC, T, P, LN,CR |
| 12 | MEP | AMT | 85 | M | 11 | 1 | 2 | L | FPC, IC, T, BG |
| 13 | MEP | AMT | 52 | F | 8 | 1 | 2 | L | C, CS, IC, T, PFL |
| 14 | MEP | RMT | 84 | M | 15 | 1 | 2 | L | CR, T, BG |
| 15 | MEP | AMT | 52 | M | 15 | 1 | 1 | R | MCA |
| 16 | MEP | AMT | 81 | F | 10 | 1 | 3 | L | MCA |
| 17 | MEP | RMT | 63 | F | 10 | 1 | 2 | R | PFL |
| 18 | MEP | RMT | 68 | F | 14 | 1 | 1 | R | PFL |
| 19 | MEP | RMT | 46 | F | 13 | 1 | 2 | L | PFL, parietal/occipital lobes, BG, T |
| 20 | no-MEP | none | none | F | 8 | 1 | NA | L | PVWM, parieto-occipal cortex |
| 21 | no-MEP | none | none | F | 8 | 1 | NA | L | LV |
| 22 | no-MEP | none | none | M | 9 | 1 | NA | R | MCA |
| 23 | no-MEP | none | none | M | 16 | 1 | NA | R | BG, C, PFL |
| 24 | no-MEP | none | none | M | 13 | 1 | NA | R | R hemisphere rostral to midbrain |
| 25 | no-MEP | none | none | F | 10 | 1 | NA | L | CR, T, PFL |
| 26 | no-MEP | none | none | M | 9 | 1 | NA | L | MCA |
| 27 | no-MEP | none | none | M | 8 | 1 | NA | L | parietal/occipital lobes |
| 28 | no-MEP | none | none | F | 9 | 1 | NA | R | BG, CS, FL, T |
| 29 | no-MEP | none | none | M | 8 | 1 | NA | L | BG, CS, MCA, FPC, IC, T |
| 30 | no-MEP | none | none | M | 8 | 2 | NA | L | CS, FPC |
| Participant | Group | M1 I/C Volume Ratio | PLIC I/C Volume Ratio | AI (%) Affected | AI (%) Less Affected | AHA Score (Logit-Based) | Mirroring |
|---|---|---|---|---|---|---|---|
| 1 | MEP | 0.863 | 0.995 | 68.23 | 73.33 | 100 | No |
| 2 | MEP | 0.839 | 0.889 | 57.10 | 64.07 | 86 | No |
| 3 | MEP | 0.918 | 0.831 | −41.03 | −40.07 | 48 | Yes |
| 4 | MEP | 0.829 | 0.403 | −13.67 | 40.83 | 35 | Yes |
| 5 | MEP | 0.986 | 0.980 | 43.50 | 64.10 | 90 | No |
| 6 | MEP | 0.830 | 0.415 | 27.30 | 48.43 | 63 | Yes |
| 7 | MEP | 0.462 | 0.898 | 66.23 | 65.10 | 67 | No |
| 8 | MEP | 0.870 | 0.681 | 53.13 | 70.87 | 66 | Yes |
| 9 | MEP | 0.301 | 0.488 | −64.30 | 73.10 | 46 | Yes |
| 10 | MEP | 0.640 | 0.789 | 46.40 | 29.23 | 67 | No |
| 11 | MEP | 0.858 | 1.110 | −15.63 | 54.00 | 54 | No |
| 12 | MEP | 0.820 | 0.778 | 7.77 | 68.63 | 59 | No |
| 13 | MEP | 0.578 | 1.022 | −52.37 | 36.23 | 57 | Yes |
| 14 | MEP | 1.070 | 0.777 | 1.40 | 42.60 | 66 | Yes |
| 15 | MEP | 0.416 | 0.753 | 57.83 | 78.47 | 55 | Yes |
| 16 | MEP | 0.526 | 0.632 | −17.72 | 43.00 | 45 | Yes |
| 17 | MEP | 1.070 | 0.707 | 42.33 | 64.20 | 71 | Yes |
| 18 | MEP | 1.130 | 0.722 | −3.83 | MD | 48 | No |
| 19 | MEP | 0.516 | 0.763 | 53.67 | 72.53 | 73 | No |
| 20 | no-MEP | 0.761 | 0.649 | −8.67 | −25.17 | NA | No |
| 21 | no-MEP | 0.106 | 0.161 | 2.07 | 21.03 | NA | Yes |
| 22 | no-MEP | 0.373 | 0.139 | −0.27 | 6.30 | NA | Yes |
| 23 | no-MEP | 0.675 | 0.536 | 44.37 | 72.76 | NA | Yes |
| 24 | no-MEP | 0.000 | 0.137 | −49.70 | 27.23 | NA | Yes |
| 25 | no-MEP | 0.847 | 0.581 | 39.63 | 49.53 | NA | Yes |
| 26 | no-MEP | 0.579 | 0.051 | 31.87 | 49.97 | NA | No |
| 27 | no-MEP | 0.436 | 0.564 | MD | MD | NA | MD |
| 28 | no-MEP | 0.228 | 0.032 | 36.13 | 40.90 | NA | No |
| 29 | no-MEP | 0.554 | 0.597 | −54.27 | −7.90 | NA | No |
| 30 | no-MEP | 0.983 | 1.058 | −6.63 | −10.07 | NA | No |
AMT active motor threshold, BG basal ganglia, C caudate, CR corona radiata, CS centrum semiovale, F female, FL frontal lobe, FPC frontoparietal cortex, GMFCS Gross Motor Function Classification Scale, GP globus pallidus, IC internal capsule, L left, LN lentiform nucleus, LV adjacent to lateral ventricle, M male, MACS Manual Abilities Classification Scale, MCA middle cerebral artery distribution, MEP ipsilesional motor-evoked potential, NA not available, no-MEP absent ipsilesional motor-evoked potential, P putamen, PFL posterior frontal lobe, PVWM periventricular white matter, R right, RMT resting motor threshold, T thalamus, WM white matter, y years
AHA Assisting Hand Assessment (Logit-Based Scores), AI accuracy index, I/C ipsilesional/contralesional, M1 primary motor cortex, MD missing data, MEP motor-evoked potential, NA not available, no-MEP absent ipsilesional motor-evoked potential, PLIC posterior limb of internal capsule
Fig. 1.
One coronal and two transverse views of anatomical magnetic resonance images depicting damage to primary motor cortex and/or posterior limb of internal capsule of participants with an ipsilesional primary motor cortex motor-evoked potential (denoted by asterisk) and those participants without a motor-evoked potential.
Table 2.
Group Comparisons
| Measure | MEP (n= 19) | no-MEP (n= 11) | p |
|---|---|---|---|
| Age (years) | 10.9 (2.87) | 9.6 (2.58) | 0.240 |
| Sex (M/F) | (10/9) | (7/4) | 0.708 |
| Stroke Hemisphere (R/L) | (6/13) | (4/7) | 1.000 |
| Mirroring during fMRI (yes/no) | (10/9) | (5/5) | 1.000 |
| Ipsilesional/Contralesional Volume Ratio | |||
| Primary Motor Cortex | 0.764 (0.240) | 0.504 (0.310) | 0.028 |
| Posterior Limb of Internal Capsule | 0.770 (0.195) | 0.410 (0.326) | 0.005 |
| Finger Tracking Accuracy Index Scores (%) | |||
| Affected Hand | 16.64 (42.19) | 3.45 (35.41) | 0.407 |
| Less Affected Hand | 52.70 (27.46) | 22.45 (31.45) | 0.016 |
Values presented as mean (standard deviation).
Fig. 2.
Demonstration of two 30-second trials of finger tracking from the less affected hand in a representative participant from the no motor-evoked potential group (no-MEP, a) and the MEP group (b). The blue line represents the target waveform with the participant's corresponding tracking responses denoted by the red line. Accuracy Index (AI) scores were normalized to each participant's maximum flexion (Max Flex.) and extension (Max Ext.) active range of motion at the metacarpophalangeal joint of the affected (not shown) and less affected index fingers. Higher positive AI scores (maximum AI score = 100%) indicate better tracking performance.
As part of a subanalysis, we examined associations between finger tracking performance and M1 and PLIC I/C volume ratios. All correlation coefficients were statistically non-significant – affected hand AI and M1 I/C ratio: r = 0.207, p = 0.282; less affected hand AI and M1 I/C ratio:ρ= 0.056, p= 0.778; affected hand AI and PLIC I/C ratio: r = 0.128, p = 0.508; and less affected hand AI and PLIC I/C ratio: ρ = 0.158, p = 0.423. An identical analysis performed exclusively on the MEP group also showed non-significant associations between affected hand AI and M1 I/C ratio: r = 0.090, p = 0.715; less affected hand A1 and M1 I/C ratio:ρ = −0.237, p = 0.345; affected hand AI and PLIC I/C ratio: r = 0.182, p = 0.455; and less affected hand AI and PLIC I/C ratio: ρ = −0.014, p = 0.958.
Participants in the MEP group who qualified for the aforementioned larger CIMT/rTMS study completed additional hand function testing involving the AHA (Table 1). A significant correlation was observed for logit-based AHA scoresand affected hand AI scores (r = 0.715, p < 0.001). The correlation between logit-based AHA scores and less affected hand AI scores was not significant (ρ = 0.316, p = 0.201).
4.Discussion
This study compared finger tracking performance and anatomical regions of interest in children with congenital hemiparesis with an elicitable vs. non-elicitable ipsilesional MEP. The important main finding was that finger tracking performance from the less affected hand, but not the affected hand, was significantly lower in participants with no MEP. Additionally, the M1 and PLIC I/C volume ratios were significantly lower in participants with no MEP.
The finger tracking task in our study required participants to visually process a target trajectory and coordinate finger extension/flexion motion to match the trajectory. We chose this task over other dexterity tasks because it is an fMRI compatible task that probes sensorimotor regions of interest (Carey et al., 2002; Deng et al., 2012; Kimberley et al., 2004; Plow et al., 2009). Previous studies have examined finger tracking ability along with other motor tests including auditory reaction time, velocity of ballistic arm movements, finger tapping, and diadochokinesis in healthy children to study motor development with respect to CST maturation assessed with TMS (Fietzek et al., 2000; Heinen et al., 1998). Investigators found that the most robust period of finger tracking development occurred within the first ten years of life, continuing beyond CST maturation and adolescent timeframes, later plateauing in early adulthood. Significant intra-individual effects for handedness (i.e. dominant vs. non-dominant hand) were also discovered (Fietzek et al., 2000). This study was the first to compare tracking performance in children with congenital hemiparesis on the basis of MEP presentation. Our unexpected yet intriguing finding was the significant difference in tracking performance of the less affected hand favoring participants with an MEP over participants with no MEP.
One potential explanation is that participants with no ipsilesional MEP relied more heavily on contralesional M1 to control the affected hand via ipsilateral CST pathways. As previously mentioned, the activity-dependent withdrawal of ipsilateral CST projections during typical development leads to contralateral motor control organization. A neurological injury like stroke may disrupt this withdrawal process thereby promoting ipsilateral motor control (Eyre et al., 2001; Martin & Lee, 1999). The possibility exists that with substantial stroke damage to the PLIC substrate, through which the CST projections descend, a favorable adaptation for the affected hand follows. The postulated adaptation in individuals with still developing nervous systems is that rather than ipsilateral CST projections from contralesional M1 withdrawing, they persist and enlarge to form a substrate for preserving at least a modicum of ipsilateral sensorimotor control of the affected hand. Indeed, even participants with the largest infarcts (Fig. 1) demonstrated at least 10 degrees of finger extension, as this was an inclusion criterion. We must surmise that because of the near total loss of cortical tissue in the stroke hemisphere in some participants (e.g. participant 24), that the observed motion in the more affected hand was likely due to ipsilateral control from the contralesional hemisphere. Unfortunately, because of excessive head movements in nearly all of the participants that invalidated their fMRI, we cannot confirm this postulate and so it remains speculative, but very worthy of future study.
In a longitudinal study combining neuroimaging and TMS to study CST developmentin infants and children, Eyre et al.(2007) found ipsilesional MEP responses in infants with unilateral lesions that later disappeared by 12 months in approximately half. Corresponding imaging revealed significantly greater growth in diameter of ipsilateral CST projections from the contralesional hemisphere compared to healthy age-matched individuals and to those with bilateral lesions. Importantly, the ipsilateral CST projections from the contralesional hemisphere in the individuals with unilateral lesions who had no ipsilesional MEP were significantly larger than the corresponding projections of the individuals with unilateral lesions who did have an ipsilesional MEP. Thus, this stroke-induced alteration in CST anatomy may reflect an ipsilateral motor control adaptation with the contralesional hemisphere contributing to voluntary movements of the affected extremities in individuals with substantial unilateral stroke damage (Eyre et al., 2007).
However, based on our finding of reduced tracking performance in the less affected hand of participants with no MEP, it appears that the postulated favorable adaptation for the more affected hand concomitantly has a maladaptive corollary consistent with the assertion by Cramer et al.(2011) that neuroplasticity can sometimes have negative consequences. The tradeoff in preserving some affected hand function may be the relinquishment of neural substrate initially dedicated to less affected hand function.
The term “crowding”, introduced by Juenger et al. (2013), refers to the competition arising when one hemisphere contains motor representations of both sides of the body. Crowding may account for the differences in tracking performance observed from the less affected hand between MEP and no-MEP groups. In those participants with an ipsilesional MEP response and a greater PLIC I/C volume ratio, there may be greater contralesional CST substrate available to control the less affected hand because of no adaptive need for “crowding” to preserve function in the more affected hand. We acknowledge that additional investigation of activity-dependent competition between corticospinal systems, specifically between the ipsilateral and contralateral fiber volumes descending from contralesional M1, is necessary to substantiate our theory.
Notably, our findings did not support our original hypothesis of significant between-group differences in affected hand finger tracking performance. In contrast, prior work has demonstrated significant relationships between corticospinal organization and the severity of affected hand impairment (Bleyenheuft, et al., 2007; Mackey et al., 2014; Rickards et al., 2014; Weinstein et al., 2013). A lack of significance may be partially explained by the multiple systems involved in tracking, and considerable uncertainty exists in the degree of motor system contribution to tracking. Hence, finger-tracking accuracy may not be as robust of a motor measure compared to other more commonly utilized upper-extremity motor tests. Despite the significant correlation between affected finger tracking and AHA, a more purely motor-based task may have better captured affected hand discrepancies between groups.
Relatedly, participants with MEPs showed increased M1 and PLIC I/C volume ratios, consistent with the literature (Duque et al., 2003; Holmström et al., 2011; Kuhnke et al., 2008; Staudt et al., 2002). However, no significant associations between I/C volume and finger tracking performance were found even after isolating the MEP group and repeating the analysis. Several studies that assessed CST and/or PLIC integrity in children using DTI technology found significant correlations with upper-extremity motor function (Bleyenheuft et al., 2007; Mackey et al., 2014; Rickards et al., 2014; Weinstein et al., 2013). The capability of these DTI measures in predicting rehabilitative gains and overall outcome requires additional investigation (Friel et al., 2014; Kirton et al., 2007; Kuhnke et al., 2008; Mackey et al., 2014; Rickards et al., 2014). The small sample size and resulting low power of this study may have prohibited the detection of significant relationships between tracking performance and volume of preserved neural substrate. Other white matter regions may also depict stronger correlations with motor function. Previous studies have found significant associations between cerebral peduncle (Bleyenheuft et al., 2007; Duque et al., 2003; Friel et al., 2014), corpus callosum (Weinstein et al., 2013), corticofugal fiber (Holmström et al., 2011) and sensorimotor thalamic projections (Rose et al., 2011) integrity with upper-extremity function. Lastly, it is possible that our I/C volume ratio method is a less sensitive measure compared to DTI. Bleyenheuft et al. (2007) measured cerebral peduncle cross-sectional area using conventional MRIs and DTI to compute asymmetry indices. Investigators discovered significant associations between these measures and with upper-extremity function, but they found that the conventional MRI method strongly underestimated the degree of CST dysgenesis (Bleyenheuft et al., 2007).
Collectively, the clinical implication of this work is that judicious consideration of the individual is key when determining post-stroke rehabilitative therapies. Based on our finger tracking results, participating in an intervention study involving low-frequency rTMS to suppress contralesional M1 excitability may be detrimental to participants with no ipsilesional MEP since it is likely that their contralesional hemisphere controls both contralateral (less affected) and ipsilateral (affected) hand function. Therefore, suppressing contralesional M1 may hinder, not enhance, affected hand function. Additionally, contralesional M1 may not be the optimal therapeutic target for rTMS delivery, aimed at suppressing cortical excitability, for participants with no MEP; thus, discouraging the “one size fits all” approach to rTMS intervention (Bradnam et al., 2012). Fittingly, the proposed bimodal-balance recovery model by Di Pino et al. (2014) emphasizes structural reserve (e.g. CST and M1 preservation) when predicting the type of brain reorganization likely shaping an individual's post-stroke recovery. Though, we recognize that the term reorganization is not applicable in congenital stroke because the nervous system is not fully mature at the time of insult. In our participants, structural reserve was contingent on MEP responses which provided a partial representation of CST integrity. MEP responses, therefore, have the potential to help guide the development of treatment parameters, therapeutic targets, and delivery while serving as potentially useful predictors of intervention responsiveness. Future studies should embrace the heterogeneity in MEP responses in stroke by focusing on effective therapeutic targets and interventions specifically for individuals showing absent ipsilesional MEPs. Combining neuroimaging with TMS testing will provide valuable insight to individual brain (re)organization following stroke that will ultimately strengthen the clinical-decision making process necessary for the skilled delivery of interventions including rTMS.
4.1 Limitations
We acknowledge a number of limitations in our study. We previously discussed how movement artifact prohibited DTI and BOLD signal examination. We believe that our methodology of using PLIC and M1 volumes from anatomical MRIs, however, is a valuable clinical measure based on similar methodology used in previous studies (Bleyenheuft et al., 2007; Duque et al., 2003). Though DTI is fast-becoming a standard tool in stroke research and may be worthwhile in the clinic setting especially in children with CP with normal-appearing MRIs (Benini et al., 2013) or combined with other measures (Shiran et al., 2014), DTI is not without its own limitations (Cheng et al., 2012).
Our study population exhibited heterogeneity in lesion size, location, and hemisphere. We also enrolled subjects with congenital hemiparesis resulting from ischemic stroke occurring before, during, or one year after birth. Such a wide developmental timeframe, encompassing gestational and postnatal periods, pose varying organizational strategies and functional outcomes dependant on the time of infarct (Staudt et al., 2002). Also, due to the observational nature of this study, participants with no MEP did not complete additional testing that included the Canadian Occupational Performance Measure, AHA, Manual Abilities Classification Scale, and stereognosis testing. Despite finding significant correlations between the affected hand finger tracking AI scores with AHA performance, an examination of finger tracking with validated unimanual dexterity tests like the Box and Block (Mathiowetz et al., 1985) and the 9-Hole Peg Test (Smith & Hong, 2000) is needed.
A comparison of finger tracking AI scores between our participants with healthy age-matched controls would provide additional insight. Former work from our lab examined this identical tracking task in 76 right-handed healthy children aged 8-9 years (Carey et al., 2003). AI scores from these children compared to our participants were greater, thus, underscoring the effect of impairment on tracking performance. Yet, tracking performance from the less affected hand in participants with an MEP was greater than tracking performances from the dominant and non-dominant hands in the healthy cohort, which may represent an age effect in tracking since our sample was, on average, older. Direct comparisons between these studies are difficult considering the discrepancies in age range, sample size, and experimental conditions related to participant positioning (sitting vs. supine in an MRI coil) and screen size, for example.
This study was part of a larger study that applied 6 Hz primed 1 Hz rTMS with behavioral therapy (CIMT) in children with congenital hemiparesis (Gillick et al., 2014). The initial approved protocol did not permit any additional pre-test TMS stimulation beyond ipsilesional M1 since study exclusion was based on the absence of an ipsilesional MEP response. Thus, an absent ipsilesional MEP does not directly correspond to ipsilateral motor organization. A bilateral MEP response from contralesional M1 would justify ipsilateral motor control organization. However, we are confident that in those children with absent ipsilesional M1 responses, affected hand function is likely subserved by other motor and/or motor-related substrates as all participants without an MEP displayed some magnitude of voluntary movement in their affected MCP joint.
4.2 Conclusion
We observed that for our sample of children with congenital hemiparesis, specifically participants with no ipsilesional MEP compared to those with an ipsilesional MEP, a reduction in finger tracking performance from the less affected hand and I/C volume ratio of M1 and PLIC. Past studies have shown activity-dependent competition in the visual (Wiesel & Hubel, 1963) and motor (Nudo, 1996) systems. We speculate that diminished tracking performance from the less affected hand may be a consequence of competition between various motor and motor-related tracts driven by both the neural injury and by maturation of the nervous system. Specifically, in more severely injured developing brains, some CST substrate from the contralesional hemisphere normally destined for the contralateral less affected hand, may be competitively redistributed to the ipsilateral more affected hand to preserve some function there, but at the expense of some function in the less affected hand. These findings suggest careful attention to the amount of neural substrate preservation when determining the optimal rTMS delivery approach to promote recovery of hand function following stroke.
Highlights.
Superior finger tracking in less affected hand seen in children with vs. without an ipsilesional motor-evoked potential (MEP) response.
Finger tracking performance from the affected hand did not differ amongst children with vs. without an ipsilesional MEP response.
Greater M1 and PLIC preservation found in children with an ipsilesional MEP response compared to those without.
Finger tracking scores were not related to neural substrate preservation.
Acknowledgements
This project received support from the National Institute of Health (1RC1HD063838-01) and from the National Center for Research Resources to the University of Minnesota Clinical and Translational Science Institute (CTSI) (1UL1RR033183). Drs. Cassidy and Gillick received support from the Foundation for Physical Therapy. Dr. Gillick received additional support from the American Academy of Cerebral Palsy. We further extend our gratitude to William Thomas, PhD and doctoral physical therapy graduate students Brian Battista, Chris Brown, Jaime Calmes, and Jaime Cupit. We are especially grateful for the dedicated children and families involved in our research.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare that they have no conflict of interest.
References
- Benini R, Dagenais L, Shevell MI. Normal imaging in patients with cerebral palsy: What does it tell us? J Pediatr. 2013;162(2):369–374. e1. doi: 10.1016/j.jpeds.2012.07.044. [DOI] [PubMed] [Google Scholar]
- Bhatt E, Nagpal A, Greer KH, Grunewald TK, Steele JL, Wiemiller JW, Carey JR. Effect of finger tracking combined with electrical stimulation on brain reorganization and hand function in subjects with stroke. Exp Brain Res. 2007;182(4):435–447. doi: 10.1007/s00221-007-1001-5. [DOI] [PubMed] [Google Scholar]
- Bleyenheuft Y, Grandin CB, Cosnard G, Olivier E, Thonnard JL. Corticospinal dysgenesis and upper-limb deficits in congenital hemiplegia: A diffusion tensor imaging study. Pediatrics. 2007;120(6):e1502–11. doi: 10.1542/peds.2007-0394. doi:10.1542/peds.2007-0394. [DOI] [PubMed] [Google Scholar]
- Bradnam LV, Stinear CM, Barber PA, Byblow WD. Contralesional hemisphere control of the proximal paretic upper limb following stroke. Cereb Cortex. 2012;22(11):2662–2671. doi: 10.1093/cercor/bhr344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey JR, Baxter TL, Di Fabio RP. Tracking control in the nonparetic hand of subjects with stroke. Arch Phys Med Rehab. 1998;79(4):435–441. doi: 10.1016/s0003-9993(98)90146-0. [DOI] [PubMed] [Google Scholar]
- Carey JR, Kimberley TJ, Lewis SM, Auerbach EJ, Dorsey L, Rundquist P, Ugurbil K. Analysis of fMRI and finger tracking training in subjects with chronic stroke. Brain. 2002;125(4):773–788. doi: 10.1093/brain/awf091. [DOI] [PubMed] [Google Scholar]
- Carey JR. Manual stretch: Effect on finger movement control and force control in stroke subjects with spastic extrinsic finger flexor muscles. Arch Phys Med Rehab. 1990;71(11):888–894. [PubMed] [Google Scholar]
- Carey JR, Durfee WK, Bhatt E, Nagpal A, Weinstein SA, Anderson KM, Lewis SM. Comparison of finger tracking versus simple movement training via telerehabilitation to alter hand function and cortical reorganization after stroke. Neurorehab Neural Re. 2007;21(3):216–232. doi: 10.1177/1545968306292381. doi:1545968306292381 [pii] [DOI] [PubMed] [Google Scholar]
- Carey JR, Evans CD, Anderson DC, Bhatt E, Nagpal A, Kimberley TJ, Pascual-Leone A. Safety of 6-hz primed low-frequency rTMS in stroke. Neurorehab Neural Re. 2008;22(2):185–192. doi: 10.1177/1545968307305458. [DOI] [PubMed] [Google Scholar]
- Carey JR, Comnick KT, Lojovich JM, Lindgren BR. Left-versus right-hand tracking performance by right-handed boys and girls: Examination of performance asymmetry. Percept Motor Skill. 2003;97(3):779–788. doi: 10.2466/pms.2003.97.3.779. [DOI] [PubMed] [Google Scholar]
- Carr LJ, Harrison LM, Evans AL, Stephens JA. Patterns of central motor reorganization in hemiplegic cerebral palsy. Brain. 1993;116(5):1223–1247. doi: 10.1093/brain/116.5.1223. [DOI] [PubMed] [Google Scholar]
- Cheeran B, Talelli P, Mori F, Koch G, Suppa A, Edwards M, Rothwell JC. A common polymorphism in the brain-derived neurotrophic factor gene (BDNF) modulates human cortical plasticity and the response to rTMS. J Physiol. 2008;586(23):5717–5725. doi: 10.1113/jphysiol.2008.159905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H, Wang Y, Sheng J, Sporns O, Kronenberger WG, Mathews VP, Saykin AJ. Optimization of seed density in DTI tractography for structural networks. J Neurosci Meth. 2012;203(1):264–272. doi: 10.1016/j.jneumeth.2011.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer SC, Sur M, Dobkin BH, O'Brien C, Sanger TD, Trojanowski JQ, Vinogradov S. Harnessing neuroplasticity for clinical applications. Brain. 2011;134(6):1591–1609. doi: 10.1093/brain/awr039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dassonville P, Lewis SM, Zhu XH, Ugurbil K, Kim SG, Ashe J. The effect of stimulus-response compatibility on cortical motor activation. NeuroImage. 2001;13(1):1–14. doi: 10.1006/nimg.2000.0671. [DOI] [PubMed] [Google Scholar]
- Deng H, Durfee WK, Nuckley DJ, Rheude BS, Severson AE, Skluzacek KM, Carey JR. Complex versus simple ankle movement training in stroke using telerehabilitation: A randomized controlled trial. Phys Ther. 2012;92(2):197–209. doi: 10.2522/ptj.20110018. doi:10.2522/ptj.20110018 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Pino G, Pellegrino G, Assenza G, Capone F, Ferreri F, Formica D, Di Lazzaro V. Modulation of brain plasticity in stroke: A novel model for neurorehabilitation. Nat Rev Neurol. 2014;10(10):597–608. doi: 10.1038/nrneurol.2014.162. doi:10.1038/nrneurol.2014.162. [DOI] [PubMed] [Google Scholar]
- Duque J, Thonnard JL, Vandermeeren Y, Sebire G, Cosnard G, Olivier E. Correlation between impaired dexterity and corticospinal tract dysgenesis in congenital hemiplegia. Brain. 2003;126(3):732–747. doi: 10.1093/brain/awg069. [DOI] [PubMed] [Google Scholar]
- Escudero JV, Sancho J, Bautista D, Escudero M, Lopez-Trigo J. Prognostic value of motor evoked potential obtained by transcranial magnetic brain stimulation in motor function recovery in patients with acute ischemic stroke. Stroke. 1998;29(9):1854–1859. doi: 10.1161/01.str.29.9.1854. [DOI] [PubMed] [Google Scholar]
- Eyre J, Taylor J, Villagra F, Smith M, Miller S. Evidence of activity-dependent withdrawal of corticospinal projections during human development. Neurology. 2001;57(9):1543–1554. doi: 10.1212/wnl.57.9.1543. [DOI] [PubMed] [Google Scholar]
- Eyre JA, Smith M, Dabydeen L, Clowry GJ, Petacchi E, Battini R, Cioni G. Is hemiplegic cerebral palsy equivalent to amblyopia of the corticospinal system?. Ann Neurol. 2007;62(5):493–503. doi: 10.1002/ana.21108. [DOI] [PubMed] [Google Scholar]
- Fietzek U, Heinen F, Berweck S, Maute S, Hufschmidt A, Schulte-Mönting J, Korinthenberg R. Development of the corticospinal system and hand motor function: Central conduction times and motor performance tests. Dev Med Child Neurol. 2000;42(4):220–227. doi: 10.1017/s0012162200000384. [DOI] [PubMed] [Google Scholar]
- Friel KM, Kuo H, Carmel JB, Rowny SB, Gordon AM. Improvements in hand function after intensive bimanual training are not associated with corticospinal tract dysgenesis in children with unilateral cerebral palsy. Exp Brain Res. 2014;232(6):2001–2009. doi: 10.1007/s00221-014-3889-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier G, Vercher J, Ivaldi FM, Marchetti E. Oculo-manual tracking of visual targets: Control learning, coordination control and coordination model. Exp Brain Res. 1988;73(1):127–137. doi: 10.1007/BF00279667. [DOI] [PubMed] [Google Scholar]
- Gillick BT, Krach LE, Feyma T, Rich TL, Moberg K, Menk J, Carey JR. Safety of primed repetitive transcranial magnetic stimulation and modified constraint-induced movement therapy in a randomized controlled trial in pediatric hemiparesis. Arch Phys Med Rehab. 2015;96(4):S104–S113. doi: 10.1016/j.apmr.2014.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillick BT, Krach LE, Feyma T, Rich TL, Moberg K, Thomas W, Carey JR. Primed low-frequency repetitive transcranial magnetic stimulation and constraint-induced movement therapy in pediatric hemiparesis: A randomized controlled trial. Dev Med Child Neurol. 2014;56(1):44–52. doi: 10.1111/dmcn.12243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinen F, Fietzek UM, Berweck S, Hufschmidt A, Deuschl G, Korinthenberg R. Fast corticospinal system and motor performance in children: Conduction proceeds skill. Pediatr Neurol. 1998;19(3):217–221. doi: 10.1016/s0887-8994(98)00057-5. [DOI] [PubMed] [Google Scholar]
- Holmström L, Lennartsson F, Eliasson A, Flodmark O, Clark C, Tedroff K, Vollmer B. Diffusion MRI in corticofugal fibers correlates with hand function in unilateral cerebral palsy. Neurology. 2011;77(8):775–783. doi: 10.1212/WNL.0b013e31822b0040. [DOI] [PubMed] [Google Scholar]
- Holmström L, Vollmer B, Tedroff K, Islam M, Persson JK, Kits A, Eliasson A. Hand function in relation to brain lesions and corticomotor-projection pattern in children with unilateral cerebral palsy. Dev Med Child Neurol. 2010;52(2):145–152. doi: 10.1111/j.1469-8749.2009.03496.x. [DOI] [PubMed] [Google Scholar]
- Juenger H, Kuhnke N, Braun C, Ummenhofer F, Wilke M, Walther M, Berweck S. Two types of exercise-induced neuroplasticity in congenital hemiparesis: A transcranial magnetic stimulation, functional MRI, and magnetoencephalography study. Dev Med Child Neurol. 2013;55(10):941–951. doi: 10.1111/dmcn.12209. [DOI] [PubMed] [Google Scholar]
- Khedr EM, Ahmed MA, Fathy N, Rothwell JC. Therapeutic trial of repetitive transcranial magnetic stimulation after acute ischemic stroke. Neurology. 2005;65(3):466–468. doi: 10.1212/01.wnl.0000173067.84247.36. [DOI] [PubMed] [Google Scholar]
- Kimberley TJ, Lewis SM, Auerbach EJ, Dorsey LL, Lojovich JM, Carey JR. Electrical stimulation driving functional improvements and cortical changes in subjects with stroke. Exp Brain Res. 2004;154(4):450–460. doi: 10.1007/s00221-003-1695-y. [DOI] [PubMed] [Google Scholar]
- Kirton A, Chen R, Friefeld S, Gunraj C, Pontigon AM, Deveber G. Contralesional repetitive transcranial magnetic stimulation for chronic hemiparesis in subcortical paediatric stroke: A randomised trial. Lancet Neurol. 2008;7(6):507–513. doi: 10.1016/S1474-4422(08)70096-6. [DOI] [PubMed] [Google Scholar]
- Kirton A, Deveber G, Gunraj C, Chen R. Cortical excitability and interhemispheric inhibition after subcortical pediatric stroke: Plastic organization and effects of rTMS. Clin Neurophysiol. 2010;121(11):1922–1929. doi: 10.1016/j.clinph.2010.04.021. [DOI] [PubMed] [Google Scholar]
- Kirton A, Shroff M, Visvanathan T, deVeber G. Quantified corticospinal tract diffusion restriction predicts neonatal stroke outcome. Stroke. 2007;38(3):974–980. doi: 10.1161/01.STR.0000258101.67119.72. doi:10.1161/01.STR.0000258101.67119.72. [DOI] [PubMed] [Google Scholar]
- Koh TH, Eyre JA. Maturation of corticospinal tracts assessed by electromagnetic stimulation of the motor cortex. Arch Dis Child. 1988;63(11):1347–52. doi: 10.1136/adc.63.11.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krumlinde-Sundholm L, Holmefur M, Kottorp A, Eliasson AC. The assisting hand assessment: Current evidence of validity, reliability, and responsiveness to change. Dev Med Child Neurol. 2007;49(4):259–264. doi: 10.1111/j.1469-8749.2007.00259.x. [DOI] [PubMed] [Google Scholar]
- Krumlinde-Sundholm L. Reporting outcomes of the assisting hand assessment: What scale should be used? Dev Med Child Neurol. 2012;54(9):807–808. doi: 10.1111/j.1469-8749.2012.04361.x. [DOI] [PubMed] [Google Scholar]
- Kuhnke N, Juenger H, Walther M, Berweck S, Mall V, Staudt M. Do patients with congenital hemiparesis and ipsilateral corticospinal projections respond differently to constraint-induced movement therapy? Dev Med Child Neurol. 2008;50(12):898–903. doi: 10.1111/j.1469-8749.2008.03119.x. [DOI] [PubMed] [Google Scholar]
- Liepert J, Zittel S, Weiller C. Improvement of dexterity by single session low-frequency repetitive transcranial magnetic stimulation over the contralesional motor cortex in acute stroke: A double-blind placebo-controlled crossover trial. Restor Neurol Neuros. 2007;25(5-6):461–465. [PubMed] [Google Scholar]
- Mackey A, Stinear C, Stott S, Byblow WD. Upper limb function and cortical organization in youth with unilateral cerebral palsy. Front Neurol. 2014;5 doi: 10.3389/fneur.2014.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda F, Keenan JP, Tormos JM, Topka H, Pascual-Leone A. Modulation of corticospinal excitability by repetitive transcranial magnetic stimulation. Clin Neurophysiol. 2000;111(5):800–805. doi: 10.1016/s1388-2457(99)00323-5. [DOI] [PubMed] [Google Scholar]
- Martin JH, Lee SJ. Activity-dependent competition between developing corticospinal terminations. Neuroreport. 1999;10(11):2277–2282. doi: 10.1097/00001756-199908020-00010. [DOI] [PubMed] [Google Scholar]
- Mathiowetz V, Federman S, Wiemer D. Box and block test of manual dexterity: Norms for 6–19 year olds. Can J Occup Ther. 1985;52(5):241–245. [Google Scholar]
- Nezu A, Kimura S, Uehara S, Kobayashi T, Tanaka M, Saito K. Magnetic stimulation of motor cortex in children: Maturity of corticospinal pathway and problem of clinical application. Brain Dev. 1997;19(3):176–80. doi: 10.1016/s0387-7604(96)00552-9. [DOI] [PubMed] [Google Scholar]
- Nudo RJ. Use-dependent alterations of movement representations in primary motor cortex of adult squirrel monkeys. J Neurosci. 1996;16(2):785. doi: 10.1523/JNEUROSCI.16-02-00785.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plow EB, Carey JR, Nudo RJ, Pascual-Leone A. Invasive cortical stimulation to promote recovery of function after stroke: A critical appraisal. Stroke. 2009;40(5):1926–1931. doi: 10.1161/STROKEAHA.108.540823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pocock SJ. Clinical trials with multiple outcomes: A statistical perspective on their design, analysis, and interpretation. Control Clin Trials. 1997;18(6):530–545. doi: 10.1016/s0197-2456(97)00008-1. [DOI] [PubMed] [Google Scholar]
- Rickards T, Sterling C, Taub E, Perkins-Hu C, Gauthier L, Graham M, Uswatte G. Diffusion tensor imaging study of the response to constraint-induced movement therapy of children with hemiparetic cerebral palsy and adults with chronic stroke. Arch Phys Med Rehab. 2014;95(3):506–514. e1. doi: 10.1016/j.apmr.2013.08.245. [DOI] [PubMed] [Google Scholar]
- Rose S, Guzzetta A, Pannek K, Boyd R. MRI structural connectivity, disruption of primary sensorimotor pathways, and hand function in cerebral palsy. Brain Connect. 2011;1(4):309–316. doi: 10.1089/brain.2011.0034. [DOI] [PubMed] [Google Scholar]
- Schaechter JD, Perdue KL, Wang R. Structural damage to the corticospinal tract correlates with bilateral sensorimotor cortex reorganization in stroke patients. NeuroImage. 2008;39(3):1370–1382. doi: 10.1016/j.neuroimage.2007.09.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seniów J, Bilik M, Leśniak M, Waldowski K, Iwański S, Członkowska A. Transcranial magnetic stimulation combined with physiotherapy in rehabilitation of poststroke hemiparesis: A randomized, double-blind, placebo-controlled study. Neurorehab Neural Re. 2012;26(9):1072–1079. doi: 10.1177/1545968312445635. [DOI] [PubMed] [Google Scholar]
- Shiran S, Weinstein M, Sirota-Cohen C, Myers V, Bashat DB, Fattal-Valevski A, Schertz M. MRI–Based radiologic scoring system for extent of brain injury in children with hemiplegia. Am J Neuroradiol. 2014;35(12):2388–2396. doi: 10.3174/ajnr.A3950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith YA, Hong E. Normative and validation studies of the nine-hole peg test with children. Percept Motor Skill. 2000;90(3):823–843. doi: 10.2466/pms.2000.90.3.823. [DOI] [PubMed] [Google Scholar]
- Staudt M, Grodd W, Gerloff C, Erb M, Stitz J, Krgeloh-Mann I. Two types of ipsilateral reorganization in congenital hemiparesis: A TMS and fMRI study. Brain. 2002;125(10):2222–37. doi: 10.1093/brain/awf227. [DOI] [PubMed] [Google Scholar]
- Stinear CM, Barber PA, Smale PR, Coxon JP, Fleming MK, Byblow WD. Functional potential in chronic stroke patients depends on corticospinal tract integrity. Brain. 2007;130(1):170–180. doi: 10.1093/brain/awl333. [DOI] [PubMed] [Google Scholar]
- Weinstein M, Green D, Geva R, Schertz M, Fattal-Valevski A, Artzi M, Gross-Tsur V. Interhemispheric and intrahemispheric connectivity and manual skills in children with unilateral cerebral palsy. Brain Struct Funct. 2013;219(3):1025–1040. doi: 10.1007/s00429-013-0551-5. [DOI] [PubMed] [Google Scholar]
- Wiesel TN, Hubel DH. Single-cell responses in striate cortex of kittens deprived of vision in one eye. J Neurophysiol. 1963;26:1003–1017. doi: 10.1152/jn.1963.26.6.1003. [DOI] [PubMed] [Google Scholar]
- Yousry TA, Schmid UD, Alkadhi H, Schmidt D, Peraud A, Buettner A, Winkler P. Localization of the motor hand area to a knob on the precentral gyrus. A new landmark. Brain. 1997;120(1):141–157. doi: 10.1093/brain/120.1.141. [DOI] [PubMed] [Google Scholar]