Figure 4.
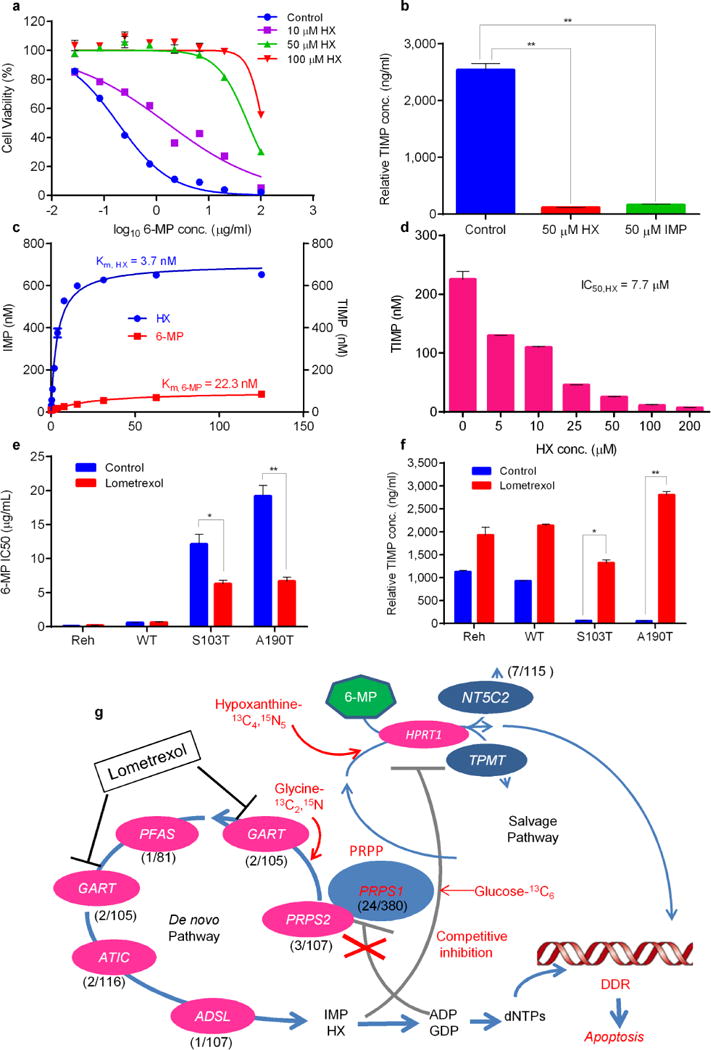
Elevated hypoxanthine (HX) directly inhibits 6-MP conversion which can be reversed by GART inhibitor lometrexol. (a) Viability of Reh cells at increasing concentrations of 6-MP in the presence of HX. (b) Reh cells were treated with 10 μM 6-MP in the presence of different nucleotides, then intracellular TIMP was measured by LC-MS. P values were calculated using two-tailed Student’s t tests (*P ≤ 0.05, **P ≤ 0.005) (c, d) HX is a preferred to 6-MP as an HPRT1 substrate and can suppress 6-MP prodrug conversion. HPRT1 enzyme assays are described in online methods, the concentration of reaction product (IMP, TIMP) was measured by LC-MS. (e, f) Viability of Reh cells with or without 5 ng/ml GART inhibitor lometrexol. TIMP was measured in the same sample set by LC-MS. P values were calculated using two-tailed Student’s t tests (*P ≤ 0.01, **P ≤ 0.001) (g) Genomics, metabolomics and enzyme data were integrated in an indirect metabolic model of thiopurine resistance in ALL involving reduced feedback inhibition, enhanced de novo biosynthesis and competitive inhibition of prodrug conversion. Resistance is reversed by GART inhibitor lometrexol, which can block de novo purine synthesis. The key genes of the de novo purine biosynthesis pathway are shown, with their mutation frequency in relapsed ALL. The mutation frequency of PRPS1, PRPS2, GART, PFAS, ATIC and ADSL was estimated by targeted sequencing of available samples (range, 81–160) from Chinese individuals with relapsed B-ALL or T-ALL. Data are shown as the means ± s.d.
