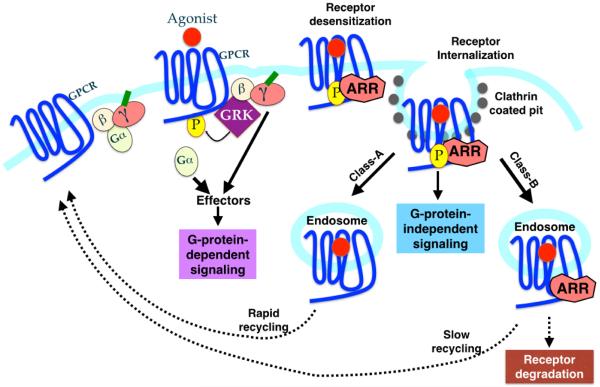
Fig 1. Classical and New Roles of Arrestins in GPCR lifecycle.
Activation of GPCR occurs by binding of the agonist, which stimulates heterotrimeric G-proteins. This G-protein-dependent signaling results in the production of second messengers. Simultaneously, GRKs phoshorylate the receptor leading to the arrestin binding and inhibition of G-protein-dependent signaling (desensitization). In addition to desensitizing the receptor by uncoupling the receptor from G-proteins, arrestins also recruit clathrin and adaptin molecules to target the desensitized receptor to clathrin coated pit. Thus arrestins play a crucial role in receptor internalization. Arrestins bound to the phosphorylated GPCRs also serve as a scaffolding platform for the MAPK modules (MAPKKK-MAPKK-MAPK). This results in the prevention of nuclear translocation of the MAPK, leading to an increase in phosphorylation of the cytosolic targets. This causes the “second-wave” of signaling, that is G-protein-independent. Affinity and duration of arrestin interaction with endocytosed GPCRs has also led to a classification of receptors into class A and B. See text for details.