Abstract
Children who experience chronic stressors are vulnerable to emotional and physical health problems across the lifespan. This phenomenon raises questions for scientists and clinicians alike. How does adversity get “under the skin” of the developing child? Through what mechanisms does it confer vulnerability to a heterogeneous set of mental and physical illnesses? And how does it instantiate risk across different life stages, engendering vulnerability to conditions that develop shortly after stressor exposure – like depression – and conditions which manifest decades later, like heart disease? Although answers to these questions have started to emerge, research has typically focused on single diseases or organ systems. To understand the plethora of health problems associated with childhood adversity, we argue that the field needs a second generation of research that recognizes multidirectional transactions amongst biological systems. To help facilitate this process, we propose a neuro-immune network hypothesis as a heuristic framework for organizing knowledge from disparate literatures, and as a springboard for generating integrative research. Drawing on existing data, we argue that early-life adversity amplifies crosstalk between peripheral inflammation and neural circuitries subserving threat-, reward-, and executive control-related processes. This crosstalk results in chronic low-grade inflammation, thereby contributing to adiposity, insulin resistance, and other predisease states. In the brain, inflammatory mediators act on cortico-amygdala threat, and cortico-basal ganglia reward, circuitries in a manner that predisposes individuals to self-medicating behaviors like smoking, drug use, and consumption of high-fat diets. Acting in concert with inflammation, these behaviors accelerate the pathogenesis of emotional and physical health problems.
Keywords: Stress, Maltreatment, Poverty, Depression, Inflammation, Heart Disease
Children who experience severe chronic stressors are vulnerable to a plethora of health problems across the lifespan (1). These problems span the continuum of what are traditionally understood to be mental and physical illnesses. For example, children maltreated by their parents not only go on to develop psychiatric disorders like unipolar depression and substance abuse at higher-than-expected rates (2), but also show increased prevalence of metabolic syndrome, coronary heart disease, some cancers, and autoimmune conditions as they age (3, 4). Children raised in families of low socioeconomic status (SES) experience disproportionately high rates of many of these same conditions (5, 6). Of course, these results derive from observational studies, which cannot by themselves distinguish between causes, effects, and confounds. But their results converge with experimental research in animals documenting long-term health consequences of early stressor exposure (7–9). Considered together, these preclinical and observational studies suggest that childhood adversity contributes to subsequent health problems in a causal manner.
This phenomenon raises challenging questions. How does adversity get “under the skin” to influence the anatomy and physiology of the developing child? Through what mechanisms does it confer vulnerability to such a heterogeneous set of health problems, spanning the continuum of mental and physical illnesses? And how does it instantiate risk across the lifespan, engendering vulnerability to conditions that develop shortly after stressor exposure – like depression – and those which manifest many decades later, like heart disease?
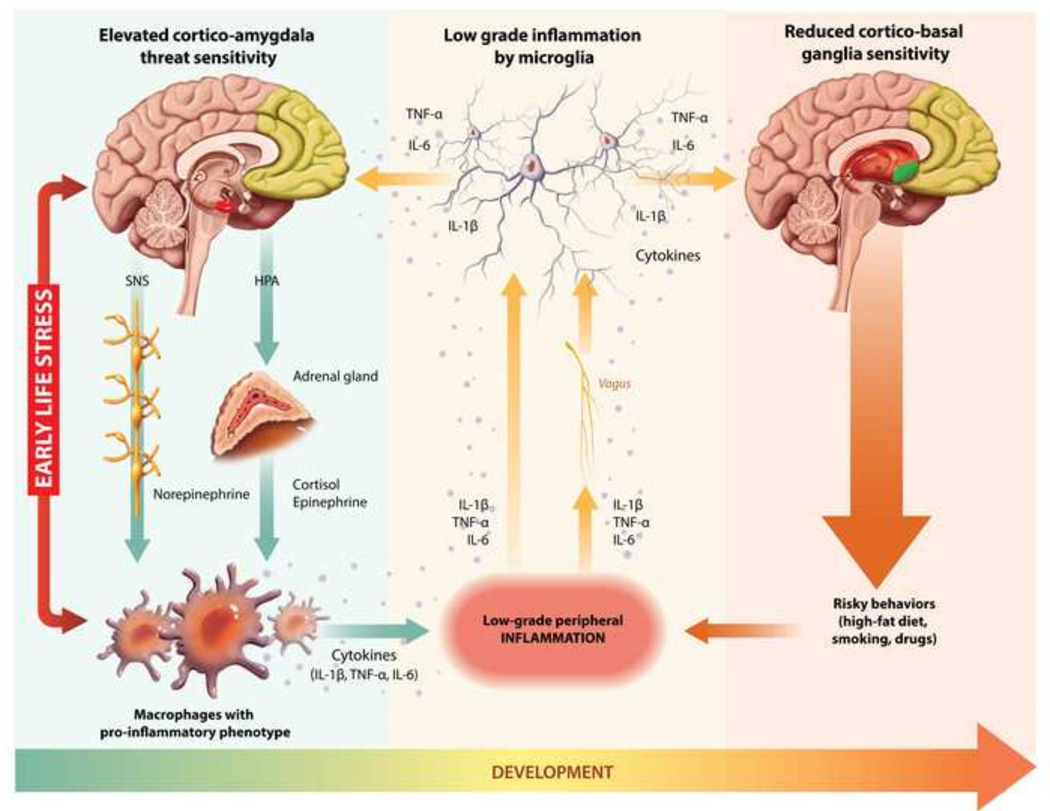
Answers to these questions have started to emerge. Over the past ten years, studies have revealed that childhood adversity is associated with subtle differences in multiple aspects of neural, cardiovascular, neuroendocrine, and immune functioning (1, 3, 4). But nearly all this work has focused on single diseases or organ systems. To understand the plethora of health problems associated with childhood adversity, the field needs a second generation of research that recognizes the multidirectional transactions amongst these systems (10). Here we attempt to begin this process, synthesizing knowledge of early adversity with recent discoveries that highlight crosstalk between the nervous and immune systems (11, 12), and establish inflammation’s role in common health problems (13, 14). As a heuristic framework, we propose a neuro-immune network hypothesis (see Figure 1). This hypothesis is not a definitive mechanistic account. Indeed, some of the proposed linkages are quite speculative, based on preclinical observations yet to be corroborated in humans. It is offered as a framework to organize knowledge from disparate literatures, and as a springboard for generating integrative research.
Figure 1.
Depiction of neuro-immune network hypothesis. Illustration by Chi-Chun Liu and Qingyang Chen. SNS = sympathetic nervous system; HPA = hypothalamic pituitary adrenocortical; IL = interleukin; TNF = tumor necrosis factor
The paper is organized into sections that represent the major tenets of the hypothesis. We begin by describing recent discoveries about stress-related changes in the nervous and immune systems. First, we discuss work showing that early adversity sensitizes the brain’s networked cortico-amygdala regions in a manner that heightens vigilance for, and reactions to, threatening stimuli. Second, we describe studies indicating that early adversity also sensitizes the immune cells that propagate inflammation (monocytes and macrophages), programming them to mount exaggerated responses to infections and injuries. Next, we review evidence that low-grade inflammation spreads to the brain. The mediators of this inflammation, cytokines, accentuate threat-related processes in the cortico-amygdala circuit, and attenuate reward-related processes in the cortico-basal ganglia circuit. Cytokines may also dampen executive control-related processes linked to regions of the prefrontal cortex. Based on these observations, we postulate the existence of multiple bidirectional pathways linking peripheral inflammation with neural circuitries subserving threat, reward, and executive control. Drawing on recent studies, we suggest that early adversity amplifies bidirectional crosstalk within these neuro-immune pathways. These exchanges result in low-grade, chronic, inflammation, which then acts on neural circuitries to facilitate self-medicating behaviors, like smoking, drug use, and consumption of high-fat and high-sugar diets. Acting in concert with inflammation, these behaviors contribute to common health problems.
Most research we describe focuses on parental maltreatment, early deprivation, or low socioeconomic status. We recognize these are heterogeneous stressors, which likely have somewhat distinct behavioral, neural, and immunologic sequelae. But to date, the limited available evidence suggests the neural-immune consequences of these stressors are more similar than different (1), so we treat them as such. We also recognize these stressors are not the only adversities that children face. War, recurrent bullying, and family instability could have similar effects, but presently, little is known about how these stressors affect processes of interest here.
Early Adversity Sensitizes Threat Vigilance and Response Systems
The first proposed neuro-immune pathway centers around the cortico-amygdala neural circuit, which supports vigilance for, and responses to, threatening stimuli. One of the most robust findings about childhood adversity is that it sensitizes cortico-amygdala neural circuitry. Relative to controls, physically abused youth develop vigilance for facial cues that connote anger, probably because those faces signal forthcoming aggression (15–18). These proclivities cause abused youth to respond aggressively to provocation, even when it is subtle (19). Similarly, children from low-SES families tend to carefully monitor their environment for danger, and maintain a low threshold for judging situations as threatening (20, 21). When confronted with ambiguous stimuli, whose threat value is uncertain, low-SES youth exhibit larger cardiovascular responses than higher-SES youth (22).
Though multiple brain systems are involved in vigilance for and response to threats, current thinking emphasizes the importance of the amygdala, and its regulation by the prefrontal cortex (PFC) (23, 24). Consistent with the notion that adversity influences how this circuitry develops, morphometric studies have documented smaller amygdala volumes in maltreated (25) and disadvantaged (26, 27) youth. In functional neuroimaging (fMRI) studies, maltreated youth show greater amygdala reactivity to emotional stimuli relative to controls (28–31) (Although see 32 for contrary findings.) This enhanced reactivity may stem from inadequate recruitment of prefrontal regions that provide top-down regulation. In a study of resting-state activity, previously maltreated adolescents showed less functional coupling of the subgenual anterior cingulate cortex (ACC) and the amygdala (33), relative to controls. Similarly, in a study of young adults, childhood SES covaried with cortico-amygdala responsivity during emotion regulation. To the extent they were reared in low-income families, subjects displayed larger amygdala responses and lower activation in both ventrolateral and dorsolateral PFC regions (34), even adjusting for current income. These findings suggest that childhood adversity’s effects on cortico-amygdala function persist into adulthood.
There are regional variations in the maturational timing of the cortico-amygdala circuit. The amygdala matures during childhood and adolescence, whereas prefrontal regions continue to develop through early adulthood (35, 36). Furthermore, prefrontal regulation of the amygdala typically emerges in adolescence (37, 38). The development of this functional coupling, however, is accelerated in children exposed to adversity (39), perhaps as a mechanism to compensate for, and down-regulate, the enhanced amygdala reactivity, and the downstream threat-response systems it mobilizes, including the sympathetic nervous system (SNS) and hypothalamic-pituitary-adrenocortical (HPA) axis. As noted below, the hormonal products of these systems – catecholamines and glucocorticoids – are modulators of inflammation.
Early Adversity Sensitizes Cells that Propagate Inflammation
In a separate literature, research shows that childhood adversity also sensitizes the immune cells that initiate and sustain inflammation. This work shows that maltreated and disadvantaged children are disproportionately exposed to pollutants, second-hand smoke, and high-fat and high-sugar diets, along with psychosocial stressors like family instability, insensitive caregiving, and neighborhood violence (40, 41). These exposures prime monocytes and macrophages to respond aggressively to pathogen-associated and danger-associated molecular patterns (1, 42). (The priming mechanisms are still being established, but likely involve some combination of direct influences, e.g., through pollutants, and indirect influences mediated through hormonal products of the SNS and HPA axis.) Indeed, to the extent they are from lower-SES families, children produce larger volumes of inflammatory cytokines when their cells are stimulated ex vivo with microbial products (43, 44), and this sensitization remains evident in adulthood (45). The monocytes of low-SES adolescents are also relatively insensitive to inhibition by glucocorticoids, which play a key physiologic role in regulating inflammation (46). Similar patterns are evident in youth reared in harsh family climates. In a longitudinal study, adolescents from harsh families showed an increasingly pro-inflammatory phenotype over 18 months. This phenotype was marked by progressively larger ex vivo cytokine responses to lipopolysaccharide (LPS), a molecule on the surface of Gram-negative bacteria. Youth from harsh families also showed declining glucocorticoid sensitivity over time; in ex vivo studies, cortisol became progressively less effective at suppressing LPS-evoked cytokine production (47). This insensitivity to glucocorticoids may be adaptive during acute threats, but if sustained would facilitate the kind of low-grade chronic inflammation implicated in many emotional and physical health problems (1, 48).
Consistent with this notion, maltreated children display higher levels of inflammatory biomarkers relative to controls, including C-reactive protein (CRP) and interleukin-6 (IL-6) (49, 50), and so do low-SES youth (51–53). This low-grade inflammation persists into adulthood; multiple studies report that inflammatory biomarkers are elevated in adults exposed to maltreatment and/or disadvantage during childhood (54–58). These associations are typically independent of adult’s concurrent psychosocial and socioeconomic conditions, suggesting that childhood adversity leaves an inflammatory “residue.”
Early Adversity Potentiates Crosstalk between Threat Circuitry and Immune System
The cortico-amygdala and inflammatory sequelae of childhood adversity have been considered in separate literatures. However, growing evidence suggests both are components of an integrated, bidirectional, network that detects threats to well-being, and mobilizes behavioral, physiologic, and inflammatory resources for coping (11, 59, 60). Next, we draw upon preclinical research to discuss how traffic might flow through this network in a bidirectional manner, and eventually become locked into a self-perpetuating cycle.
Brain-to-Immune Traffic
Research has identified the anatomical basis of brain-to-immune signaling (61). When stimuli are perceived as threatening and uncontrollable, cell groups in the amygdala signal hypothalamic centers that subsequently mobilize fight-or-flight responses mediated by the SNS and HPA axis (62). SNS fibers descend from the brain into organs where leukocytes mature, including bone marrow, the spleen, and thymus, and into organs where pathogen-leukocyte interactions transpire, like the lymph nodes, gut, and lungs (63). Under threat conditions, these fibers release norepinephrine onto resident monocytes and macrophages, activating an inflammatory gene expression program that causes these cells to migrate towards damaged tissue and aggressively release mediators that accelerate pathogen removal and wound healing (11). As this unfolds, neural threat circuits also signal the adrenal gland to boost systemic output of epinephrine and glucocorticoids. These hormones initially reinforce norepinephrine’s pro-inflammatory actions, but as the threat subsides they counter-regulate it (61, 64). From an evolutionary perspective, this brain-to-immune signaling is likely adaptive, enabling the organism to mobilize and sensitize cells in anticipation of a forthcoming attack, during which injury and infection are likely. Doing so would presumably speed pathogen eradication and tissue healing.
Immune-to-Brain Traffic
Research shows that network traffic also flows the other direction, from immune to brain. Peripheral inflammation can spread to the brain through multiple mechanisms. Cytokines, like IL-1β, IL-6, and TNF-α, can access the brain through active transport, or can enter at circumventricular organs or leaky regions of the blood-brain-barrier. Peripheral cytokines can also engage receptors on afferent vagal fibers, which project to limbic regions via the nucleus of the solitary tract (11, 12). This immune-to-brain traffic can modulate cortico-amygdala circuitry involved in threat processing. In rodents, repeated social defeat causes peripheral monocytes to migrate into the parenchyma of multiple brain regions, including the amygdala, hippocampus, and PFC. Stress causes these monocytes, along with tissue-resident microglia, to express pro-inflammatory mediators in the brain. Stress also has a longer-lasting priming effect, sensitizing microglia so the next time they encounter a pathogen, the inflammatory response is larger (65–68). This stress-evoked neuro-inflammatory response occurs in parallel with anxiety-like behaviors, including increased light-dark preference and decreased open-field exploration. Notably, mice lacking a functional receptor for IL-1β, a key inflammatory mediator, do not exhibit defeat-evoked microglial activation or anxiety behaviors (68). These results suggest a role for the neuro-inflammatory response in threat processing.
One human study suggests similar effects of inflammation on cortico-amygdala functioning. Healthy adults underwent fMRI following administration of low-dose LPS or placebo. LPS-treated subjects displayed larger amygdala responses to threatening images (69). From an evolutionary perspective, this signaling could be adaptive, enabling peripheral leukocytes to send “warning signals” to the brain, which act through cortico-amygdala pathways to enhance threat-vigilance. The ensuing behavioral adjustments could prevent or reduce exposure to stimuli likely to cause injury or infection.
Self-Perpetuating Cycle?
The findings above indicate that childhood adversity sensitizes cortico-amygdala circuitry that subserve threat processing, and the monocytes and macrophages that propagate inflammation. As a result, these systems overshoot threat responses, generating increased traffic through the neuro-immune network. We speculate that over time this traffic takes on a life of its own, creating a positive-feedback circuit where signals flow in both directions, from brain-to-immune and from immune-to-brain. Whether such a positive-feedback circuit actually exists is unknown, and is an important question for subsequent research. Even if it does not, the functional connections between these systems are likely to result in cross-sensitization (11, 70), wherein larger neural threat responses, acting via the SNS and HPA axis, amplify inflammation, and vice-versa. On its own, this cross-sensitization would presumably increase network traffic and, if our broader hypothesis is accurate, heighten risk for later health problems. Next, we expand this discussion to include links between adversity, inflammation, and reward- and executive control-related neural circuitries.
Early Adversity Potentiates Crosstalk between Reward Circuitry and Immune System
Another potential bidirectional pathway in the neuro-immune network involves the cortico-basal ganglia circuit, which supports reward processing (71). This circuit involves projections from midbrain nuclei (e.g., substantia nigra), to subcortical areas within the basal ganglia (e.g., ventral striatum) and cortical target regions (e.g., orbito-medial frontal cortex). Dopamine is the neurotransmitter most directly involved in reward processing within this circuit, playing a central role in incentive motivation, reward-based learning, and motor control (71).
Early Adversity and Reward Sensitivity
Similar to the cortico-amygdala circuit, growing evidence indicates that early adversity affects development of the cortico-basal ganglia circuit and the reward-related behaviors it subserves. Animals exposed to early-life stress display weakened preferences for sweet foods, higher thresholds for brain stimulation reward, and blunted responses to amphetamines and dopamine agonists (72–74). Convergent with these findings, research in humans indicates that maltreatment is associated with later reward-processing deficits on behavioral tasks (75–76). fMRI studies are elucidating the neural bases of these associations, and show that relative to controls, previously maltreated individuals have decreased activation in the basal ganglia (ventral striatum, globus pallidus) following monetary gains (76, 77). Because these studies were performed years after maltreatment, they suggest a persistent effect on reward-related brain function. Parallel findings have emerged in those from disadvantaged backgrounds. In an fMRI study of monetary gains, adults from low-SES families showed reduced activation in the dorsal-medial PFC and ACC, and less functional connectivity between these areas and striatal regions implicated in reward processing (78). Further research is needed to substantiate these findings, and clarify the underlying mechanisms (e.g., dopamine transmission).
Inflammation, Reward, and Depression
Though early adversity undoubtedly influences reward sensitivity through multiple pathways, growing evidence suggests a possible mechanistic role for inflammation. Blunted reward sensitivity is part of a generalized set of adaptations to infection, mediated by inflammatory cytokines (79, 80). These adaptations are collectively referred to as sickness behaviors, and along with anhedonia, include dysphoria, fatigue, psychomotor slowing, and inactivity (81). Sickness behaviors have evolutionary adaptive qualities, maximizing an organism’s chances of surviving infection by diverting energetic resources to the immune system, and minimizing contact with other pathogens and predators (80).
In line with this perspective, research demonstrates that inflammatory mediators reduce animals’ sensitivity to rewarding stimuli, including reinforcers like sex, food, and electrical stimulation (81). Inflammatory products also promote tolerance to the reinforcing properties of many drugs, including opiates, cocaine, alcohol, and amphetamines (82). Experimental studies in humans also suggest a role for inflammation in modulating reward sensitivity. One fMRI study found that low-dose LPS reduced ventral striatal reactivity to monetary gain (83). Another found that a typhoid vaccine, by eliciting peripheral inflammation, affected neural responses to an emotion perception task. Compared to placebo, typhoid-treated subjects showed disrupted functional connections between the subgenual ACC and the ventral striatum, among other subcortical regions involved in reward processing (84). Other research has focused on patients with chronic hepatitis C, who are treated with the immune-activating cytokine interferon-a. After 4–6 weeks of treatment, these patients displayed reduced ventral striatal activation in response to gambling wins. This deficit was secondary to blunted dopamine transmission in the basal ganglia (85).
Inflammation’s effects extend beyond reward sensitivity to depression more generally. Multiple prospective studies have found that inflammatory biomarkers forecast depressive symptoms and episodes (86–88). In patients who receive inflammatory cytokines for treatment of hepatitis C or malignant melanoma, rates of depression are 30–50 percent (89, 90). However, the excess depression risks associated with these agents can be ameliorated by pretreatment with paroxetine (89, 90). Moreover, a recent clinical trial showed that a cytokine antagonist – infliximab – reduces depressive symptoms in treatment-resistant patients with elevated inflammatory markers (91).
Interestingly, the associations between inflammation and depression are especially strong in persons exposed to childhood adversity. Danese and colleagues stratified young adults into subgroups based upon a history of childhood maltreatment and past-year major depression. Inflammation was higher among maltreated, depressed subjects, relative to controls with neither. Depression alone was not associated with inflammation (92). Similarly, a six-wave study of adolescents found that inflammation presaged the development of syndromal depression. But this association was only present in subjects exposed to relatively high levels of previous adversity (88).
Implications for Poor Health-Related Behaviors
We hypothesize that by dampening reward sensitivity and increasing dysphoric feelings, inflammation facilitates high-risk behaviors with negative health consequences. Across the lifecourse, childhood maltreatment is associated with higher rates of cigarette smoking, excessive alcohol consumption, drug misuse, physical inactivity, and high-fat eating (93). The same trends are seen with low childhood SES (1). Our thinking here is grounded in the reward deficiency model of addiction, which postulates that persons with low reward sensitivity self-medicate negative emotions and/or attempt to elevate positive and reward-related emotions through high-risk behaviors (94–96). Consistent with this model, preclinical research documents that blunted dopamine signaling in the basal ganglia is centrally involved in many addictive behaviors, including food-seeking and obesity (95–99). In humans, cause-and-effect relationships are less clear. However, preliminary findings from neurogenetic research indicates that reduced reward-related brain function in the striatum may reflect both a pre-existing vulnerability for, as well as a consequence of, engaging in high-risk behaviors (100), suggesting a bidirectional relationship between dampened reward sensitivity and addictive/poor-health behaviors. Future research is needed to more fully unpack these links.
Many high-risk behaviors linked to blunted reward sensitivity have downstream effects on inflammation. Indeed, smoking promotes inflammation (101), as does consumption of high-fat and high-sugar foods (102), and sedentary behavior (103). The latter behaviors contribute to weight gain, which itself is a potent inflammatory stimulus (104). If the inflammation triggered by risky behaviors spreads to the brain, it could modulate cortico-basal ganglia circuitry to further blunt reward sensitivity. This would create a second bidirectional pathway in the neuro-immune network, coupling reward circuitry, risky behavior, and inflammation. Whether a positive-feedback circuit of this nature exists is unknown. But if so, it could help explain why childhood adversity confers vulnerability to the array of inflammation-dependent health problems (see below).
Reduced Prefrontal Regulation Maintains Neuro-Immune Network
As implied, we believe that deficiencies in prefrontal executive control also contribute to neuro-immune network activity. The PFC supports a variety of affective and cognitive processes. Medial PFC regions have been implicated in incentive/risk processing, social cognition, and response inhibition, whereas lateral PFC regions are principally involved in higher executive functions, impulse control, and voluntary emotion regulation (105). These functions are implemented, in part, through top-down influences on threat and reward circuitries, coordinated via white matter fiber tracts from the medial PFC and ACC to the amygdala and basal ganglia, amongst other regions (106). As noted, the PFC has a relatively protracted maturational timeline, which renders it particularly vulnerable to early-life adversity. Indeed, structural neuroimaging studies indicate that multiple forms of early adversity (low SES, physical abuse, emotional abuse, neglect, harsh punishment) are associated with reduced grey matter volume, cortical thickness, and white matter tract integrity in both medial and lateral PFC regions (25, 26, 106–109). fMRI studies report that early-life adversity is associated with reduced prefrontal activation during laboratory tasks, and atypical connectivity between the amygdala and both the medial PFC and subgenual ACC (33, 39, 110). Furthermore, prefrontal abnormalities mediate the relationship between early adversity and executive control deficits (107).
Although studied less extensively than the amygdala and basal ganglia, there is growing evidence that inflammation modulates PFC structure, function, and development in a manner that diminishes executive control and self-regulation. Experimental animal models demonstrate that inflammation can slow myelination (111) and, in humans, inflammatory biomarkers are associated with smaller medial PFC volume and reduced cortical white matter integrity (112, 113). One study found that low-SES adults had reduced whole-brain white matter integrity, and that inflammation partially mediated this relationship (106). fMRI studies demonstrate that administration of inflammatory stimuli, like cytokines and vaccines, modulate activity of the dorsolateral PFC, and both the subgenual and dorsal ACC (84, 85). Inflammation also modulates subgenual ACC regulation of threat and reward circuitries discussed above (84). Future research is needed to clarify the mechanisms and boundaries of these associations.
Consequences for Emotional and Physical Health
As noted, children exposed to severe adversity are prone to a plethora of health problems across the lifespan (1–4). All of these conditions develop through etiologically complex transactions between genetics, lifestyle, and the environment, as mediated via dysregulation of multiple physiological systems. Yet over the past decade, it is become apparent that chronic, low-grade inflammation is a “common soil” that helps fertilize the development and progression of many of them (14). These conditions range from traditional psychiatric disorders, like depression and substance misuse, to allergic, metabolic, cardiovascular, neoplastic, and rheumatic diseases (12, 13, 114, 115). Notably, there is much overlap between this list of conditions with a suspected inflammatory etiology, and the catalog of health problems disproportionately prevalent among persons who experienced childhood adversity. Noting this overlap, we hypothesize that the proposed neuro-immune network fuels chronic low-grade inflammatory activity, which acts together with genetics, lifestyle, and other exposures to accelerate the pathogenesis of health problems. Because of space limitations, we cannot detail the connections amongst childhood adversity, inflammation, and disease pathogenesis here. But Table 1 highlights key literature documenting these associations.
Table 1.
Health problems across the lifespan whose onset and/or course may be affected by early adversity and subsequent activation of the proposed neuro-immune network
| Health Problem | Early Adversity and Onset and/or Course |
Inflammation’s Role in Pathogenesis |
|---|---|---|
| Early – Middle Childhood | ||
| Asthma | (21, 119, 120) | (114) |
| Respiratory Infection | (121–122) | (123) |
| Adolescence – Early Adulthood | ||
| Depression | (88, 124, 125) | (79) |
| Substance Misuse | (126–128) | (129–130) |
| Obesity | (131–132) | (104) |
| Insulin Resistance | (133–135) | (136) |
| Preclinical Atherosclerosis | (137–138) | (139) |
| Middle – Later Adulthood | ||
| Metabolic Syndrome | (117, 140–141) | (142) |
| Stroke | (143–145) | (142) |
| Coronary Heart Disease | (139, 143, 145, 146) | (142) |
| Selected Cancers | (93, 147, 148) | (115) |
| Autoimmune Conditions | (149, 150) | (14) |
Note. Because of space limitations, we are unable to cite many relevant studies here. Instead, we cite exemplar studies and definitive reviews. We apologize to authors whose work was omitted.
Limitations and Concluding Remarks
We argue that understanding how childhood adversity presages health problems across the lifespan requires a fresh, integrated approach to research. Accordingly, we have articulated a neuro-immune network hypothesis and considered evidence for its basic tenets. Although each tenet is grounded in evidence, additional research is needed to determine whether they unfold and interact in the manner the hypothesis specifies. That research will have to combine preclinical studies, which can further elucidate underlying mechanisms, and multiwave longitudinal investigations, which trace children’s psychosocial, neural, and inflammatory trajectories across development. As knowledge from these endeavors accumulates, the hypothesis will need to be revised accordingly. Some missing pieces, like genetic influences, are already evident, while others await relevant evidence. For instance, the hypothesis assumes that distinct adversities, like maltreatment and disadvantage, have roughly similar neural and immune repercussions. But as knowledge accrues we may need to revisit this assumption, and formulate adversity-specific pathways. In future research the issue of resilience will also have to be considered. Even in populations of children who experience severe adversity, only a minority go on to develop any specific health problem (116). Resources like parental nurturance appear to function as stress buffers for these youth (117), and subsequent versions of the hypothesis will have to acknowledge these moderators and clarify how they operate. The network’s developmental timeline must also be elucidated. Based on knowledge of how these systems mature (35, 36), we suspect that adversity’s effects on cortico-amygdala and inflammatory functioning manifest in childhood, whereas influences on reward- and executive-functions come online later, during adolescence and adulthood. But this hypothesis will need to be tested explicitly in longitudinal studies. Despite these limitations, the framework provides a prospectus to guide future research, and highlights candidate pathways for interventions with high-risk youth.
Acknowledgments
Preparation of this article was supported by grants from the National Institute of Mental Health (R01 MH100117), National Institute on Drug Abuse (P30 DA0278270), and National Heart, Lung, and Blood Institute (R01 HL 122328).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures
Both authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Miller GE, Chen E, Parker KJ. Psychological stress in childhood and susceptibility to the chronic diseases of aging: Moving toward a model of behavioral and biological mechanisms. Psychol Bull. 2011;137:959–997. doi: 10.1037/a0024768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Teicher MH, Samson JA. Childhood maltreatment and psychopathology: A case for ecophenotypic variants as clinically and neurobiologically distinct subtypes. Am J Psychiatry. 2013;170:1114–1133. doi: 10.1176/appi.ajp.2013.12070957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Danese A, McEwen BS. Adverse childhood experiences, allostasis, allostatic load, and age-related disease. Physiol Behav. 2012;106:29–39. doi: 10.1016/j.physbeh.2011.08.019. [DOI] [PubMed] [Google Scholar]
- 4.Shonkoff JP, Boyce WT, McEwen BS. Neuroscience, molecular biology, and the childhood roots of health disparities: building a new framework for health promotion and disease prevention. JAMA. 2009;301:2252–2259. doi: 10.1001/jama.2009.754. [DOI] [PubMed] [Google Scholar]
- 5.Galobardes B, Lynch JW, Smith GD. Is the association between childhood socioeconomic circumstances and cause-specific mortality established? Update of a systematic review. J Epidemiol Community Health. 2008;62:387–390. doi: 10.1136/jech.2007.065508. [DOI] [PubMed] [Google Scholar]
- 6.Reiss F. Socioeconomic inequalities and mental health problems in children and adolescents: a systematic review. Soc Sci Med. 2013;90:24–31. doi: 10.1016/j.socscimed.2013.04.026. [DOI] [PubMed] [Google Scholar]
- 7.Avitsur R, Hunzeker J, Sheridan JF. Role of early stress in the individual differences in host response to viral infection. Brain Behav Immun. 2006;20:339–348. doi: 10.1016/j.bbi.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 8.Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10:434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- 9.Andersson IJ, Jiang YY, Davidge ST. Maternal stress and development of atherosclerosis in the adult apolipoprotein E-deficient mouse offspring. Am J Physiol Regul Integr Comp Physiol. 2009;296:R663–R671. doi: 10.1152/ajpregu.90768.2008. [DOI] [PubMed] [Google Scholar]
- 10.McEwen BS, Gianaros PJ. Central role of the brain in stress and adaptation: links to socioeconomic status, health, and disease. Ann N Y Acad Sci. 2010;1186:190–222. doi: 10.1111/j.1749-6632.2009.05331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Irwin MR, Cole SW. Reciprocal regulation of the neural and innate immune systems. Nat Rev Immunol. 2011;11:625–632. doi: 10.1038/nri3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haroon E, Raison CL, Miller AH. Psychoneuroimmunology meets neuropsychopharmacology: translational implications of the impact of inflammation on behavior. Neuropsychopharmacology. 2012;37:137–162. doi: 10.1038/npp.2011.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nathan C, Ding A. Nonresolving inflammation. Cell. 2010;140:871–882. doi: 10.1016/j.cell.2010.02.029. [DOI] [PubMed] [Google Scholar]
- 14.Scrivo R, Vasile M, Bartosiewicz I, Valesini G. Inflammation as “common soil” of the multifactorial diseases. Autoimmun Rev. 2011;10:369–374. doi: 10.1016/j.autrev.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 15.Pollak SD. Mechanisms linking early experience and the emergence of emotions: Illustrations from the study of maltreated children. Curr Dir Psychol Sci. 2008;17:370–375. doi: 10.1111/j.1467-8721.2008.00608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pollak SD, Tolley-Schell SA. Selective attention to facial emotion in physically abused children. J Abnorm Psychol. 2003;112:323–338. doi: 10.1037/0021-843x.112.3.323. [DOI] [PubMed] [Google Scholar]
- 17.Pollak SD, Sinha P. Effects of early experience on children’s recognition of facial displays of emotion. Dev Psychol. 2002;38:784–791. doi: 10.1037//0012-1649.38.5.784. [DOI] [PubMed] [Google Scholar]
- 18.Pollak SD, Kistler DJ. Early experience is associated with the development of categorical representations for facial expressions of emotion. Proc Natl Acad Sci U S A. 2002;99:9072–9076. doi: 10.1073/pnas.142165999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dodge KA. Translational science in action: hostile attributional style and the development of aggressive behavior problems. Dev Psychopathol. 2006;18:791–814. doi: 10.1017/s0954579406060391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen E, Matthews KA. Development of the cognitive appraisal and understanding of social events (CAUSE) videos. Health Psychol. 2003;22:106–110. doi: 10.1037//0278-6133.22.1.106. [DOI] [PubMed] [Google Scholar]
- 21.Chen E, Hanson MD, Paterson LQ, Griffin MJ, Walker HA, Miller GE. Socioeconomic status and inflammatory processes in childhood asthma: The role of psychological stress. Journal of Allergy and Clinical Immunology. 2006;117:1014–1020. doi: 10.1016/j.jaci.2006.01.036. [DOI] [PubMed] [Google Scholar]
- 22.Chen E, Langer DA, Raphaelson YE, Matthews KA. Socioeconomic status and health in adolescents: the role of stress interpretations. Child Dev. 2004;75:1039–1052. doi: 10.1111/j.1467-8624.2004.00724.x. [DOI] [PubMed] [Google Scholar]
- 23.Davis M, Whalen PJ. The amygdala: vigilance and emotion. Mol Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- 24.Price JL, Drevets WC. Neurocircuitry of mood disorders. Neuropsychopharmacology. 2010;35:192–216. doi: 10.1038/npp.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Edmiston EE, Wang F, Mazure CM, Guiney J, Sinha R, Mayes LC, et al. Corticostriatal-limbic gray matter morphology in adolescents with self-reported exposure to childhood maltreatment. Arch Pediatr Adolesc Med. 2011;165:1069–1077. doi: 10.1001/archpediatrics.2011.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luby J, Belden A, Botteron K, Marrus N, Harms MP, Babb C, et al. The effects of poverty on childhood brain development: the mediating effect of caregiving and stressful life events. JAMA Pediatr. 2013;167:1135–1142. doi: 10.1001/jamapediatrics.2013.3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Noble KG, Houston SM, Kan E, Sowell ER. Neural correlates of socioeconomic status in the developing human brain. Dev Sci. 2012;15:516–527. doi: 10.1111/j.1467-7687.2012.01147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tottenham N, Hare TA, Millner A, Gilhooly T, Zevin JD, Casey BJ. Elevated amygdala response to faces following early deprivation. Dev Sci. 2011;14:190–204. doi: 10.1111/j.1467-7687.2010.00971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Harmelen AL, van Tol MJ, Demenescu LR, van der Wee NJ, Veltman DJ, Aleman A, et al. Enhanced amygdala reactivity to emotional faces in adults reporting childhood emotional maltreatment. Soc Cogn Affect Neurosci. 2013;8:362–369. doi: 10.1093/scan/nss007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McCrory EJ, De Brito SA, Kelly PA, Bird G, Sebastian CL, Mechelli A, et al. Amygdala activation in maltreated children during pre-attentive emotional processing. Br J Psychiatry. 2013;202:269–276. doi: 10.1192/bjp.bp.112.116624. [DOI] [PubMed] [Google Scholar]
- 31.Dannlowski U, Kugel H, Huber F, Stuhrmann A, Redlich R, Grotegerd D, et al. Childhood maltreatment is associated with an automatic negative emotion processing bias in the amygdala. Hum Brain Mapp. 2013;34:2899–2909. doi: 10.1002/hbm.22112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taylor SE, Eisenberger NI, Saxbe D, Lehman BJ, Lieberman MD. Neural responses to emotional stimuli are associated with childhood family stress. Biol Psychiatry. 2006;60:296–301. doi: 10.1016/j.biopsych.2005.09.027. [DOI] [PubMed] [Google Scholar]
- 33.Herringa RJ, Birn RM, Ruttle PL, Burghy CA, Stodola DE, Davidson RJ, et al. Childhood maltreatment is associated with altered fear circuitry and increased internalizing symptoms by late adolescence. Proc Natl Acad Sci U S A. 2013;110:19119–19124. doi: 10.1073/pnas.1310766110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim P, Evans GW, Angstadt M, Ho SS, Sripada CS, Swain JE, et al. Effects of childhood poverty and chronic stress on emotion regulatory brain function in adulthood. Proc Natl Acad Sci U S A. 2013;110:18442–18447. doi: 10.1073/pnas.1308240110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leppanen JM, Nelson CA. Tuning the developing brain to social signals of emotions. Nat Rev Neurosci. 2009;10:37–47. doi: 10.1038/nrn2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee FS, Heimer H, Giedd JN, Lein ES, Sestan N, Weinberger DR, et al. Mental health. Adolescent mental health--opportunity and obligation. Science. 2014;346:547–549. doi: 10.1126/science.1260497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gabard-Durnam LJ, Flannery J, Goff B, Gee DG, Humphreys KL, Telzer E, et al. The development of human amygdala functional connectivity at rest from 4 to 23 years: a cross-sectional study. Neuroimage. 2014;95:193–207. doi: 10.1016/j.neuroimage.2014.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gee DG, Humphreys KL, Flannery J, Goff B, Telzer EH, Shapiro M, et al. A developmental shift from positive to negative connectivity in human amygdala-prefrontal circuitry. J Neurosci. 2013;33:4584–4593. doi: 10.1523/JNEUROSCI.3446-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gee DG, Gabard-Durnam LJ, Flannery J, Goff B, Humphreys KL, Telzer EH, et al. Early developmental emergence of human amygdala-prefrontal connectivity after maternal deprivation. Proc Natl Acad Sci U S A. 2013;110:15638–15643. doi: 10.1073/pnas.1307893110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Evans GW. The environment of childhood poverty. Am Psychol. 2004;59:77–92. doi: 10.1037/0003-066X.59.2.77. [DOI] [PubMed] [Google Scholar]
- 41.Cicchetti D, Toth SL. Child maltreatment. Annu Rev Clin Psychol. 2005;1:409–438. doi: 10.1146/annurev.clinpsy.1.102803.144029. [DOI] [PubMed] [Google Scholar]
- 42.Rook GA, Raison CL, Lowry CA. Microbial ‘old friends’, immunoregulation and socioeconomic status. Clin Exp Immunol. 2014;177:1–12. doi: 10.1111/cei.12269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Azad MB, Lissitsyn Y, Miller GE, Becker AB, HayGlass KT, Kozyrskyj AL. Influence of socioeconomic status trajectories on innate immune responsiveness in children. PLoS ONE. 2012;7:e38669. doi: 10.1371/journal.pone.0038669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wright RJ, Visness CM, Calatroni A, Grayson MH, Gold DR, Sandel MT, et al. Prenatal maternal stress and cord blood Innate and adaptive cytokine responses in an inner-city cohort. Am J Respir Crit Care Med. 2010;182:25–33. doi: 10.1164/rccm.200904-0637OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miller GE, Chen E, Fok A, Walker H, Lim A, Nicholls EP, et al. Low early-life social class leaves a biological residue manifest by decreased glucocorticoid and increased pro-inflammatory signaling. Proc Natl Acad Sci U S A. 2009;106:14716–14721. doi: 10.1073/pnas.0902971106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schreier HM, Roy LB, Frimer LT, Chen E. Family chaos and adolescent inflammatory profiles: the moderating role of socioeconomic status. Psychosom Med. 2014;76:460–467. doi: 10.1097/PSY.0000000000000078. [DOI] [PubMed] [Google Scholar]
- 47.Miller GE, Chen E. Harsh family climate in early life presages the emergence of pro-inflammatory phenotype in adolescence. Psychological Science. 2010;21:848–856. doi: 10.1177/0956797610370161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Raison CL, Miller AH. When not enough is too much: The role of insufficient glucocorticoid signaling in the pathophysiology of stress-related disorders. Am J Psychiatry. 2003;160:1554–1565. doi: 10.1176/appi.ajp.160.9.1554. [DOI] [PubMed] [Google Scholar]
- 49.Slopen N, Kubzansky LD, McLaughlin KA, Koenen KC. Childhood adversity and inflammatory processes in youth: a prospective study. Psychoneuroendocrinology. 2013;38:188–200. doi: 10.1016/j.psyneuen.2012.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Danese A, Caspi A, Williams B, Ambler A, Sugden K, Mika J, et al. Biological embedding of stress through inflammation processes in childhood. Mol Psychiatry. 2010;16:244–246. doi: 10.1038/mp.2010.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Broyles ST, Staiano AE, Drazba KT, Gupta AK, Sothern M, Katzmarzyk PT. Elevated C-reactive protein in children from risky neighborhoods: evidence for a stress pathway linking neighborhoods and inflammation in children. PLoS ONE. 2012;7:e45419. doi: 10.1371/journal.pone.0045419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schreier HM, Chen E. Socioeconomic status in one’s childhood predicts offspring cardiovascular risk. Brain Behav Immun. 2010;24:1324–1331. doi: 10.1016/j.bbi.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 53.Khandaker GM, Pearson RM, Zammit S, Lewis G, Jones PB. Association of serum interleukin 6 and C-reactive protein in childhood with depression and psychosis in young adult life: a population-based longitudinal study. JAMA Psychiatry. 2014;10:1121–1128. doi: 10.1001/jamapsychiatry.2014.1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Danese A, Moffitt TE, Harrington H, Milne BJ, Polanczyk G, Pariante CM, et al. Adverse childhood experiences and adult risk factors for age-related disease: depression, inflammation, and clustering of metabolic risk markers. Arch Pediatr Adolesc Med. 2009;163:1135–1143. doi: 10.1001/archpediatrics.2009.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Danese A, Pariante CM, Caspi A, Taylor A, Poulton R. Childhood maltreatment predicts adult inflammation in a life-course study. Proc Natl Acad Sci U S A. 2007;104:1319–1324. doi: 10.1073/pnas.0610362104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kiecolt-Glaser JK, Gouin JP, Weng NP, Malarkey WB, Beversdorf DQ, Glaser R. Childhood adversity heightens the impact of later-life caregiving stress on telomere length and inflammation. Psychosom Med. 2011;73:16–22. doi: 10.1097/PSY.0b013e31820573b6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Matthews KA, Chang YF, Thurston RC, Bromberger JT. Child abuse is related to inflammation in mid-life women: role of obesity. Brain Behav Immun. 2014;36:29–34. doi: 10.1016/j.bbi.2013.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Loucks EB, Pilote L, Lynch JW, Richard H, Almeida ND, Benjamin EJ, et al. Life course socioeconomic position is associated with inflammatory markers: the Framingham Offspring Study. Soc Sci Med. 2010;71:187–195. doi: 10.1016/j.socscimed.2010.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Miller AH, Haroon E, Raison CL, Felger JC. Cytokine targets in the brain: impact on neurotransmitters and neurocircuits. Depress Anxiety. 2013;30:297–306. doi: 10.1002/da.22084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Slavich GM, Irwin MR. From stress to inflammation and major depressive disorder: A social signal transduction theory of depression. Psychol Bull. 2014;140:774–815. doi: 10.1037/a0035302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sternberg EM. Neural regulation of innate immunity: A coordinated nonspecific host response to pathogens. Nat Rev Immunol. 2006;6:318–328. doi: 10.1038/nri1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rodrigues SM, LeDoux JE, Sapolsky RM. The influence of stress hormones on fear circuitry. Annu Rev Neurosci. 2009;32:289–313. doi: 10.1146/annurev.neuro.051508.135620. [DOI] [PubMed] [Google Scholar]
- 63.Webster JI, Tonelli L, Sternberg EM. Neuroendocrine regulation of immunity. Annu Rev Immunol. 2002;20:125–163. doi: 10.1146/annurev.immunol.20.082401.104914. [DOI] [PubMed] [Google Scholar]
- 64.Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- 65.Wohleb ES, Powell ND, Godbout JP, Sheridan JF. Stress-induced recruitment of bone marrow-derived monocytes to the brain promotes anxiety-like behavior. J Neurosci. 2013;33:13820–13833. doi: 10.1523/JNEUROSCI.1671-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Frank MG, Watkins LR, Maier SF. Stress- and glucocorticoid-induced priming of neuroinflammatory responses: potential mechanisms of stress-induced vulnerability to drugs of abuse. Brain Behav Immun. 2011;25(Suppl 1):S21–S28. doi: 10.1016/j.bbi.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Frank MG, Baratta MV, Sprunger DB, Watkins LR, Maier SF. Microglia serve as a neuroimmune substrate for stress-induced potentiation of CNS pro-inflammatory cytokine responses. Brain Behav Immun. 2007;21:47–59. doi: 10.1016/j.bbi.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 68.Wohleb ES, Hanke ML, Corona AW, Powell ND, Stiner LM, Bailey MT, et al. beta-Adrenergic receptor antagonism prevents anxiety-like behavior and microglial reactivity induced by repeated social defeat. J Neurosci. 2011;31:6277–6288. doi: 10.1523/JNEUROSCI.0450-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Inagaki TK, Muscatell KA, Irwin MR, Cole SW, Eisenberger NI. Inflammation selectively enhances amygdala activity to socially threatening images. Neuroimage. 2012;59:3222–3226. doi: 10.1016/j.neuroimage.2011.10.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Anisman H. Cascading effects of stressors and inflammatory immune system activation: implications for major depressive disorder. J Psychiatry Neurosci. 2009;34:4–20. [PMC free article] [PubMed] [Google Scholar]
- 71.Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Matthews K, Robbins TW. Early experience as a determinant of adult behavioural responses to reward: the effects of repeated maternal separation in the rat. Neurosci Biobehav Rev. 2003;27:45–55. doi: 10.1016/s0149-7634(03)00008-3. [DOI] [PubMed] [Google Scholar]
- 73.Pryce CR, Dettling AC, Spengler M, Schnell CR, Feldon J. Deprivation of parenting disrupts development of homeostatic and reward systems in marmoset monkey offspring. Biol Psychiatry. 2004;56:72–79. doi: 10.1016/j.biopsych.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 74.Phillips GD, Howes SR, Whitelaw RB, Robbins TW, Everitt BJ. Isolation rearing impairs the reinforcing efficacy of intravenous cocaine or intra-accumbens d-amphetamine: impaired response to intra-accumbens D1 and D2/D3 dopamine receptor antagonists. Psychopharmacology (Berl) 1994;115:419–429. doi: 10.1007/BF02245085. [DOI] [PubMed] [Google Scholar]
- 75.Guyer AE, Kaufman J, Hodgdon HB, Masten CL, Jazbec S, Pine DS, et al. Behavioral alterations in reward system function: the role of childhood maltreatment and psychopathology. J Am Acad Child Adolesc Psychiatry. 2006;45:1059–1067. doi: 10.1097/01.chi.0000227882.50404.11. [DOI] [PubMed] [Google Scholar]
- 76.Dillon DG, Holmes AJ, Birk JL, Brooks N, Lyons-Ruth K, Pizzagalli DA. Childhood adversity is associated with left basal ganglia dysfunction during reward anticipation in adulthood. Biol Psychiatry. 2009;66:206–213. doi: 10.1016/j.biopsych.2009.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mehta MA, Gore-Langton E, Golembo N, Colvert E, Williams SC, Sonuga-Barke E. Hyporesponsive reward anticipation in the basal ganglia following severe institutional deprivation early in life. J Cogn Neurosci. 2010;22:2316–2325. doi: 10.1162/jocn.2009.21394. [DOI] [PubMed] [Google Scholar]
- 78.Gianaros PJ, Manuck SB, Sheu LK, Kuan DC, Votruba-Drzal E, Craig AE, et al. Parental education predicts corticostriatal functionality in adulthood. Cereb Cortex. 2011;21:896–910. doi: 10.1093/cercor/bhq160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65:732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Maier SF, Watkins LR. Cytokines for psychologists: Implications of bidirectional immune-to-brain communication for understanding behavior, mood, and cognition. Psychological Review. 1998;105:83–107. doi: 10.1037/0033-295x.105.1.83. [DOI] [PubMed] [Google Scholar]
- 81.Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Coller JK, Hutchinson MR. Implications of central immune signaling caused by drugs of abuse: mechanisms, mediators and new therapeutic approaches for prediction and treatment of drug dependence. Pharmacol Ther. 2012;134:219–245. doi: 10.1016/j.pharmthera.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 83.Eisenberger NI, Berkman ET, Inagaki TK, Rameson LT, Mashal NM, Irwin MR. Inflammation-induced anhedonia: endotoxin reduces ventral striatum responses to reward. Biol Psychiatry. 2010;68:748–754. doi: 10.1016/j.biopsych.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Harrison NA, Brydon L, Walker C, Gray MA, Steptoe A, Critchley HD. Inflammation causes mood changes through alterations in subgenual cingulate activity and mesolimbic connectivity. Biol Psychiatry. 2009;66:407–414. doi: 10.1016/j.biopsych.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Capuron L, Pagnoni G, Drake DF, Woolwine BJ, Spivey JR, Crowe RJ, et al. Dopaminergic mechanisms of reduced basal ganglia responses to hedonic reward during interferon alfa administration. Arch Gen Psychiatry. 2012;69:1044–1053. doi: 10.1001/archgenpsychiatry.2011.2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Stewart JC, Rand KL, Muldoon MF, Kamarck TW. A prospective evaluation of the directionality of the depression-inflammation relationship. Brain Behav Immun. 2009;23:936–944. doi: 10.1016/j.bbi.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Matthews KA, Schott LL, Bromberger JT, Cyranowski JM, Everson-Rose SA, Sowers M. Are there bi-directional associations between depressive symptoms and C-reactive protein in mid-life women? Brain Behav Immun. 2010;24:96–101. doi: 10.1016/j.bbi.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Miller GE, Cole SW. Clustering of depression and inflammation in adolescents previously exposed to childhood adversity. Biol Psychiatry. 2012;72:34–40. doi: 10.1016/j.biopsych.2012.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Musselman DL, Lawson DH, Gumnick JF, Manatunga AK, Penna S, Goodkin RS, et al. Paroxetine for the prevention of depression induced by high-dose interferon alfa. N Engl J Med. 2001;344:961–966. doi: 10.1056/NEJM200103293441303. [DOI] [PubMed] [Google Scholar]
- 90.Capuron L, Ravaud A, Dantzer R. Early depressive symptoms in cancer patients receiving interleukin 2 and/or interferon alfa-2b therapy. J Clin Oncol. 2000;18:2143–2151. doi: 10.1200/JCO.2000.18.10.2143. [DOI] [PubMed] [Google Scholar]
- 91.Raison CL, Rutherford RE, Woolwine BJ, Shuo C, Schettler P, Drake DF, et al. A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: the role of baseline inflammatory biomarkers. JAMA Psychiatry. 2013;70:31–41. doi: 10.1001/2013.jamapsychiatry.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Danese A, Moffitt TE, Pariante CM, Ambler A, Poulton R, Caspi A. Elevated inflammation levels in depressed adults with a history of childhood maltreatment. Arch Gen Psychiatry. 2008;65:409–415. doi: 10.1001/archpsyc.65.4.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, et al. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. Am J Prev Med. 1998;14:245–258. doi: 10.1016/s0749-3797(98)00017-8. [DOI] [PubMed] [Google Scholar]
- 94.Blum K, Braverman ER, Holder JM, Lubar JF, Monastra VJ, Miller D, et al. Reward deficiency syndrome: a biogenetic model for the diagnosis and treatment of impulsive, addictive, and compulsive behaviors. J Psychoactive Drugs. 2000;32(Suppl:i-iv):1–112. doi: 10.1080/02791072.2000.10736099. [DOI] [PubMed] [Google Scholar]
- 95.Volkow ND, Fowler JS, Wang GJ. The addicted human brain: insights from imaging studies. J Clin Invest. 2003;111:1444–1451. doi: 10.1172/JCI18533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bowirrat A, Oscar-Berman M. Relationship between dopaminergic neurotransmission, alcoholism, and Reward Deficiency syndrome. Am J Med Genet B Neuropsychiatr Genet. 2005;132B:29–37. doi: 10.1002/ajmg.b.30080. [DOI] [PubMed] [Google Scholar]
- 97.Volkow ND, Fowler JS, Wang GJ, Swanson JM, Telang F. Dopamine in drug abuse and addiction: results of imaging studies and treatment implications. Arch Neurol. 2007;64:1575–1579. doi: 10.1001/archneur.64.11.1575. [DOI] [PubMed] [Google Scholar]
- 98.Volkow ND, Wang GJ, Fowler JS, Tomasi D, Baler R. Food and drug reward: overlapping circuits in human obesity and addiction. Curr Top Behav Neurosci. 2012;11:1–24. doi: 10.1007/7854_2011_169. [DOI] [PubMed] [Google Scholar]
- 99.Johnson PM, Kenny PJ. Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nat Neurosci. 2010;13:635–641. doi: 10.1038/nn.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Stice E, Spoor S, Bohon C, Small DM. Relation between obesity and blunted striatal response to food is moderated by TaqIA A1 allele. Science. 2008;322:449–452. doi: 10.1126/science.1161550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yanbaeva DG, Dentener MA, Creutzberg EC, Wesseling G, Wouters EF. Systemic effects of smoking. Chest. 2007;131:1557–1566. doi: 10.1378/chest.06-2179. [DOI] [PubMed] [Google Scholar]
- 102.Kiecolt-Glaser JK. Stress, food, and inflammation: psychoneuroimmunology and nutrition at the cutting edge. Psychosom Med. 2010;72:365–369. doi: 10.1097/PSY.0b013e3181dbf489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Handschin C, Spiegelman BM. The role of exercise and PGC1alpha in inflammation and chronic disease. Nature. 2008;454:463–469. doi: 10.1038/nature07206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 105.Phillips ML, Ladouceur CD, Drevets WC. A neural model of voluntary and automatic emotion regulation: implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Mol Psychiatry. 2008;13:829, 833–857. doi: 10.1038/mp.2008.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gianaros PJ, Marsland AL, Sheu LK, Erickson KI, Verstynen TD. Inflammatory Pathways Link Socioeconomic Inequalities to White Matter Architecture. Cereb Cortex. 2013;23:2058–2071. doi: 10.1093/cercor/bhs191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hanson JL, Chung MK, Avants BB, Rudolph KD, Shirtcliff EA, Gee JC, et al. Structural variations in prefrontal cortex mediate the relationship between early childhood stress and spatial working memory. J Neurosci. 2012;32:7917–7925. doi: 10.1523/JNEUROSCI.0307-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hanson JL, Hair N, Shen DG, Shi F, Gilmore JH, Wolfe BL, et al. Family poverty affects the rate of human infant brain growth. PLoS ONE. 2013;8:e80954. doi: 10.1371/journal.pone.0080954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lawson GM, Duda JT, Avants BB, Wu J, Farah MJ. Associations between children’s socioeconomic status and prefrontal cortical thickness. Dev Sci. 2013;16:641–652. doi: 10.1111/desc.12096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sheridan MA, Sarsour K, Jutte D, D’Esposito M, Boyce WT. The impact of social disparity on prefrontal function in childhood. PLoS ONE. 2012;7:e35744. doi: 10.1371/journal.pone.0035744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Meyer U. Developmental neuroinflammation and schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2013;42:20–34. doi: 10.1016/j.pnpbp.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 112.Marsland AL, Gianaros PJ, Abramowitch SM, Manuck SB, Hariri AR. Interleukin-6 covaries inversely with hippocampal grey matter volume in middle-aged adults. Biol Psychiatry. 2008;64:484–490. doi: 10.1016/j.biopsych.2008.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Satizabal CL, Zhu YC, Mazoyer B, Dufouil C, Tzourio C. Circulating IL-6 and CRP are associated with MRI findings in the elderly: the 3C–Dijon Study. Neurology. 2012;78:720–727. doi: 10.1212/WNL.0b013e318248e50f. [DOI] [PubMed] [Google Scholar]
- 114.Prescott SL. The development of respiratory inflammation in children. Paediatr Respir Rev. 2006;7:89–96. doi: 10.1016/j.prrv.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 115.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 116.Chen E, Miller GE. Shift and persist strategies: Why being low in socioeconomic status isn’t always bad for your health. Perspectives on Psychological Science. 2012;7:135–158. doi: 10.1177/1745691612436694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Miller GE, Lachman ME, Chen E, Gruenewald TL, Karlamangla AS, Seeman TE. Pathways to resilience: maternal nurturance as a buffer against the effects of childhood poverty on metabolic syndrome at midlife. Psychol Sci. 2011;22:1591–1599. doi: 10.1177/0956797611419170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Franken IH, Muris P, Georgieva I. Gray’s model of personality and addiction. Addict Behav. 2006;31:399–403. doi: 10.1016/j.addbeh.2005.05.022. [DOI] [PubMed] [Google Scholar]
- 119.Wright RJ, Mitchell H, Visness CM, Cohen S, Stout J, Evans R, et al. Community violence and asthma morbidity: The Inner-City Asthma Study. Am J Public Health. 2004;94:625–632. doi: 10.2105/ajph.94.4.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Shankardass K, McConnell R, Jerrett M, Milam J, Richardson J, Berhane K. Parental stress increases the effect of traffic-related air pollution on childhood asthma incidence. Proc Natl Acad Sci U S A. 2009;106:12406–12411. doi: 10.1073/pnas.0812910106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Cohen S, Janicki-Deverts D, Turner RB, Marsland AL, Casselbrant ML, Li-Korotky HS, et al. Childhood socioeconomic status, telomere length, and susceptibility to upper respiratory infection. Brain Behav Immun. 2013;34:31–38. doi: 10.1016/j.bbi.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Clover RD, Abell T, Becker LA, Crawford S, Ramsey CN. Family functioning and stress as predictors of influenza B infection. Journal of Family Practice. 1989;5:535–539. [PubMed] [Google Scholar]
- 123.Doyle WJ, Skoner DP, Gentile D. Nasal cytokines as mediators of illness during the common cold. Curr Allergy Asthma Rep. 2005;5:173–181. doi: 10.1007/s11882-005-0034-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.McLaughlin KA, Greif Green J, Gruber MJ, Sampson NA, Zaslavsky AM, Kessler RC. Childhood adversities and first onset of psychiatric disorders in a national sample of US adolescents. Arch Gen Psychiatry. 2012;69:1151–1160. doi: 10.1001/archgenpsychiatry.2011.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Elovainio M, Pulkki-Raback L, Jokela M, Kivimaki M, Hintsanen M, Hintsa T, et al. Socioeconomic status and the development of depressive symptoms from childhood to adulthood: a longitudinal analysis across 27 years of follow-up in the Young Finns study. Soc Sci Med. 2012;74:923–929. doi: 10.1016/j.socscimed.2011.12.017. [DOI] [PubMed] [Google Scholar]
- 126.Melchior M, Moffitt TE, Milne BJ, Poulton R, Caspi A. Why do children from socioeconomically disadvantaged families suffer from poor health when they reach adulthood? A life-course study. Am J Epidemiol. 2007;166:966–974. doi: 10.1093/aje/kwm155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Enoch MA. The role of early life stress as a predictor for alcohol and drug dependence. Psychopharmacology (Berl) 2011;214:17–31. doi: 10.1007/s00213-010-1916-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Widom CS, White HR, Czaja SJ, Marmorstein NR. Long-term effects of child abuse and neglect on alcohol use and excessive drinking in middle adulthood. Journal of Studies on Alcohol and Drugs. 2007;68:317–326. doi: 10.15288/jsad.2007.68.317. [DOI] [PubMed] [Google Scholar]
- 129.Hutchinson MR, Shavit Y, Grace PM, Rice KC, Maier SF, Watkins LR. Exploring the neuroimmunopharmacology of opioids: an integrative review of mechanisms of central immune signaling and their implications for opioid analgesia. Pharmacol Rev. 2011;63:772–810. doi: 10.1124/pr.110.004135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Costello EJ, Copeland WE, Shanahan L, Worthman CM, Angold A. C-reactive protein and substance use disorders in adolescence and early adulthood: a prospective analysis. Drug Alcohol Depend. 2013;133:712–717. doi: 10.1016/j.drugalcdep.2013.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Danese A, Tan M. Childhood maltreatment and obesity: systematic review and meta-analysis. Mol Psychiatry. 2014;19:544–554. doi: 10.1038/mp.2013.54. [DOI] [PubMed] [Google Scholar]
- 132.Braveman PA, Cubbin C, Egerter S, Williams DR, Pamuk E. Socioeconomic disparities in health in the United States: what the patterns tell us. Am J Public Health. 2010;100(Suppl 1):S186–S196. doi: 10.2105/AJPH.2009.166082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Goodman E, Daniels SR, Dolan LM. Socioeconomic disparities in insulin resistance: results from the Princeton School District Study. Psychosom Med. 2007;69:61–67. doi: 10.1097/01.psy.0000249732.96753.8f. [DOI] [PubMed] [Google Scholar]
- 134.Lehman BJ, Taylor SE, Kiefe CI, Seeman TE. Relation of childhood socioeconomic status and family environment to adult metabolic functioning in the CARDIA study. Psychosom Med. 2005;67:846–854. doi: 10.1097/01.psy.0000188443.48405.eb. [DOI] [PubMed] [Google Scholar]
- 135.Kivimaki M, Smith GD, Juonala M, Ferrie JE, Keltikangas-Jarvinen L, Elovainio M, et al. Socioeconomic position in childhood and adult cardiovascular risk factors, vascular structure, and function: cardiovascular risk in young Finns study. Heart. 2006;92:474–480. doi: 10.1136/hrt.2005.067108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Odegaard JI, Chawla A. Pleiotropic actions of insulin resistance and inflammation in metabolic homeostasis. Science. 2013;339:172–177. doi: 10.1126/science.1230721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Thurston RC, Chang Y, Derby CA, Bromberger JT, Harlow SD, Janssen I, et al. Abuse and subclinical cardiovascular disease among midlife women: the study of women’s health across the nation. Stroke. 2014;45:2246–2251. doi: 10.1161/STROKEAHA.114.005928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Matthews KA, Schwartz JE, Cohen S. Indices of socioeconomic position across the life course as predictors of coronary calcification in black and white men and women: coronary artery risk development in young adults study. Soc Sci Med. 2011;73:768–774. doi: 10.1016/j.socscimed.2011.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Libby P, Ridker PM, Hansson GK. Inflammation in atherosclerosis: from pathophysiology to practice. J Am Coll Cardiol. 2009;54:2129–2138. doi: 10.1016/j.jacc.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Midei AJ, Matthews KA, Chang YF, Bromberger JT. Childhood physical abuse is associated with incident metabolic syndrome in mid-life women. Health Psychol. 2013;32:121–127. doi: 10.1037/a0027891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Langenberg C, Kuh D, Wadsworth ME, Brunner E, Hardy R. Social circumstances and education: Life course origins of social inequalities in metabolic risk in a prospective national birth cohort. Am J Public Health. 2006;96:2216–2221. doi: 10.2105/AJPH.2004.049429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ridker PM. Inflammatory biomarkers and risks of myocardial infarction, stroke, diabetes, and total mortality: implications for longevity. Nutr Rev. 2007;65:S253–S259. doi: 10.1111/j.1753-4887.2007.tb00372.x. [DOI] [PubMed] [Google Scholar]
- 143.Rich-Edwards JW, Mason S, Rexrode K, Spiegelman D, Hibert E, Kawachi I, et al. Physical and sexual abuse in childhood as predictors of early-onset cardiovascular events in women. Circulation. 2012;126:920–927. doi: 10.1161/CIRCULATIONAHA.111.076877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Johnson RC, Schoeni RF. Early-life origins of adult disease: National longitudinal population-based study of the United States. Am J Public Health. 2011;101:2317–2324. doi: 10.2105/AJPH.2011.300252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Galobardes B, Smith GD, Lynch JW. Systematic review of the influence of childhood socioeconomic circumstances on risk for cardiovascular disease in adulthood. Ann Epidemiol. 2006;16:91–104. doi: 10.1016/j.annepidem.2005.06.053. [DOI] [PubMed] [Google Scholar]
- 146.Dong M, Giles WH, Felitti VJ, Dube SR, Williams JE, Chapman DP, et al. Insights into causal pathways for ischemic heart disease: Adverse childhood experiences study. Circulation. 2004;110:1761–1766. doi: 10.1161/01.CIR.0000143074.54995.7F. [DOI] [PubMed] [Google Scholar]
- 147.Keinan-Boker L, Vin-Raviv N, Liphshitz I, Linn S, Barchana M. Cancer incidence in Israeli Jewish survivors of World War II. J Natl Cancer Inst. 2009;101:1489–1500. doi: 10.1093/jnci/djp327. [DOI] [PubMed] [Google Scholar]
- 148.Lawlor DA, Sterne JA, Tynelius P, Davey Smith G, Rasmussen F. Association of childhood socioeconomic position with cause-specific mortality in a prospective record linkage study of 1,839,384 individuals. Am J Epidemiol. 2006;164:907–915. doi: 10.1093/aje/kwj319. [DOI] [PubMed] [Google Scholar]
- 149.Dube SR, Fairweather D, Pearson WS, Felitti VJ, Anda RF, Croft JB. Cumulative childhood stress and autoimmune diseases in adults. Psychosom Med. 2009;71:243–250. doi: 10.1097/PSY.0b013e3181907888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ziol-Guest KM, Duncan GJ, Kalil A, Boyce WT. Early childhood poverty, immune-mediated disease processes, and adult productivity. Proc Natl Acad Sci U S A. 2012;109(Suppl 2):17289–17293. doi: 10.1073/pnas.1203167109. [DOI] [PMC free article] [PubMed] [Google Scholar]