Abstract
Coral reefs are an indispensible worldwide resource, accounting for billions of dollars in cultural, economic, and ecological services. An understanding of coral reproduction is essential to determining the effects of environmental stressors on coral reef ecosystems and their persistence into the future. Here we describe the presence of and changes in steroidal hormones along with associated steroidogenic and steroid removal enzymes during the reproductive cycle of the brooding, pan-Pacific, hermaphroditic coral, Pocillopora damicornis. Detectable levels of 17β-estradiol, estrone, progesterone and testosterone were consistently detected over two consecutive lunar reproductive cycles in coral tissue. Intra-colony variation in steroid hormone levels ranged between 1.5 and 2.2 fold and were not statistically different. Activities of the steroidogenic enzymes 3β-hydroxysteroid dehydrogenase and cytochrome P450 (CYP) 17 dehydrogenase were detectable and did not fluctuate over the reproductive cycle. Aromatase-like activity was detected during the lunar reproductive cycle with no significant fluctuations. Activities of regeneration enzymes did not fluctuate over the lunar cycle; however, activity of the clearance enzyme UDP-glucuronosyl transferases increased significantly (ANOVA, post hoc p<0.01) during the two weeks before and after peak larval release (planulation), suggesting activity of this enzyme family may be linked to the reproductive state of the coral. Sulfotransferase enzymes could not be detected. Our findings provide the first data defining normal physiological and lunar/reproductive variability in steroidal enzymes in a coral species with respect to their potential role in coral reproduction.
Keywords: clearance, coral, enzyme, molecular reproduction, regeneration, steroid hormones, steroidogenesis
Introduction
Coral reefs play an essential role in our biosphere; providing environmental, economic and cultural resources valued in the billions of dollars (Cesar et al., 2003). A critical factor for the persistence of coral reefs is the ability of coral species to reproduce, that is, form new individuals from prior stock. Previous studies of coral reproduction have provided a wealth of information across numerous species describing precise timeframes for coral reproduction, reproductive strategies (spawning vs. brooding) and sexual mode (hermaphroditic vs. gonochoric) (Richmond and Hunter, 1990; Twan et al., 2006; Veron, 1995, 2000). Despite this array of classification and observational data, the molecular mechanisms of coral reproduction, particularly production, disposition, tissue distribution and clearance of molecules tied to reproduction, have received limited attention and remain largely unknown.
Steroid molecules are derived from the precursor cholesterol and serve a number of important physiological functions. The set of molecules collectively known as “steroid hormones,” helps mediate physiological processes such as growth, development and reproduction (Evans, 1988; Gorski and Gannon, 1976; Porterfield and White, 2007). Several steroid hormones identical to vertebrate sex hormones have been identified in members of the phylum Cnidaria. In the sea pansy, Renilla koellikeri, annual patterns of 17β-estradiol levels that coincided with the reproductive cycle were discovered (Pernet and Anctil, 2002). Several steroid hormones including estrone, 17β-estradiol, progesterone, androstenedione and testosterone have been detected in both stony and soft corals (Armoza-Zvuloni et al., 2012; Blomquist et al., 2006; Pernet and Anctil, 2002; Slattery et al., 1999; Slattery et al., 1997; Tarrant et al., 1999; Tarrant et al., 2003; Twan et al., 2003; Twan et al., 2006). Additionally, steroidogenic enzymes responsible for the production of steroid hormones, including 5α-reductase, 3β-hydroxysteroid dehydrogenase (3βHSD), 17β-hydroxysteroid dehydrogenase, aromatase and acyl transferase, have also been reported (Slattery et al., 1997; Tarrant et al., 2003; Twan et al., 2003).
The current investigation was designed to measure steroid hormones and their precursors in the hermaphroditic brooding coral, Pocillopora damicornis. Additionally, the presence and activity of associated steroidogenic, steroid elimination and steroid regeneration enzymes were also investigated to address the potential molecular mechanisms associated with coral reproduction. The activities of the steroidogenic enzymes 3βHSD, cytochrome P450 17 dehydrogenase (CYP17), and aromatase as well as the clearance enzymes UDP-glucuronosyl transferase (UGT) and sulfotransferase (SULT) and the regeneration enzymes β-glucuronidase and arylsulfatase C (ASC) have also been characterized. Moreover, a determination of natural fluctuations in these molecules was undertaken, in order to provide: 1) a description of baseline values (with error estimates), to improve the quality and effectiveness of “point-in-time” studies, since without estimates of baseline fluctuations it is difficult to determine the significance of changes at individual time-points; and 2) determine if the reproductive cycles align with measured changes. The data presented herein represent the first detailed description of the changes of these characteristics in the coral species P. damicornis over its lunar reproductive cycle. Since steroidal enzymes can also be involved in xenobiotic metabolism, it is important to understand natural fluctuations in order to accurately apply proteomic techniques to studying the effects of exogenous stressors on coral and coral reef health.
Materials and Methods
Coral collection
Whole colonies (~15 cm diameter; n = 5) of the coral P. damicornis were collected from the reef flat surrounding Coconut Island in Kaneohe Bay, Oahu under Special Activities Permit 2009-42, granted by the Hawaii Department of Land and Natural Resources, Division of Aquatic Resources. Corals were selected at random, cleaned of foreign organisms and placed in a quarantine tank for two weeks prior to introduction into flowing seawater tanks at the Kewalo Marine Laboratory. All water leaving the quarantine tank was filtered and sterilized using a SMART Ultraviolet Sterilizer Model 02025 (Emperor Aquatics Inc., Pottstown, PA, USA) prior to release. After quarantine, colonies were maintained in separate seawater tanks and allowed at least 31 days to recuperate from possible stress from collection and allowed to acclimatize to the Kewalo seawater system prior to tissue sampling. The Kewalo seawater system consists of an unfiltered open flow with an intake 300 m offshore at a depth of 10 m.
Experimental Procedure
Individual colonies (n = 5) were repeatedly sampled for two months at selected time points during the reproductive lunar cycle, defined as from one full moon to the next, approximately 29 days (Richmond and Jokiel, 1984). Sampling time points were taken at each lunar quarter, every seven to eight days, with P. damicornis planulation event occurring every 3rd quarter in the lunar cycle (Kolinski and Cox, 2003; Richmond and Jokiel, 1984). Coral branches (~7 cm tall, 2.5 cm wide) were removed from the base of the colony where the skeleton was devoid of tissue in order to avoid further stress. Samples were placed in conical 50 mL polypropylene Falcon tubes, flash frozen in liquid nitrogen and immediately placed at −80 °C until further processing.
To appropriately represent the reproductive cycle timing of P. damicornis, the data are reported as days from the start of sampling, with day 1 being the first day of sampling at the full moon. This time point also represents one week prior to planulation. The subsequent sampling time points represent the lunar quarters as follows: 3rd quarter moon (planulation event); new moon (one week after planulation event); 1st quarter moon (two weeks after planulation event), over the two-month sampling period.
Tissue Preparation of Whole Cell Lysates and Postmitochondrial subcellular fractions
Coral tissue was removed from the skeleton using a Water Pik dental cleaner and 0.2 μm filtered seawater (FSW) (Johannes and Wiebe, 1970). The resulting coral ‘blastate’ was transferred to conical 50 mL polypropylene Falcon tubes and spun at 10,000 x g for ten min at 4 °C in a Sorvall RC-5B Centrifuge using a fixed rotor (DuPont Instruments, San Pedro, CA) to pellet tissue and free floating cells. The resulting pellets were resuspended and combined in 5 mL of cold homogenization buffer (FSW; 1mM Phenylmethylsulfonylfluoride) and homogenized on ice with an Ultra-Turrax homogenizer for 60 s. The homogenate was placed into a conical 15 mL polypropylene Falcon tube and centrifuged at 2,000 x g for five min at 4 °C in an Eppendorf Centrifuge 5810R (Eppendorf, Hauppauge, NY). The zooxanthellae pellet was discarded and the supernatant was transferred to a new 15 mL Falcon tube and spun again at 2,000 x g for 3 min to remove any remaining zooxanthellae. The final supernatant, whole cell lysate (WCL), consisting primarily of coral tissue, was aliquoted and frozen at −80 °C until use. The postmitochondrial subcellular fraction of coral host protein, void of zooxanthellae, mitochondria, plasma membrane and nuclei, was obtained by processing the WCL using a glass homogenizer for 2 min (approx. 30 strokes) on ice. The resulting homogenate was centrifuging at 10,000 x g for 20 min at 4°C in an Eppendorf Microcentrifuge 5415D (Eppendorf, Hauppauge, NY). After this final centrifugation, the supernatant represents the postmitochondrial tissue fraction of protein for coral, consisting of microsomes and cytosol.
Assays for Steroid Hormones
The measurement of cholesterol and steroid hormones was performed on coral tissue WCL. All WCLs were diluted to the desired protein concentration: 0.25 mg/mL for total cholesterol, 1 mg/mL for free cholesterol and 2 mg/mL for steroid hormone ELISA assays using the BCA method (Smith et al., 1985). Total and free cholesterol were measured using biochemical assay kits as per the manufacturer's instructions (Cayman Chemical, Ann Arbor, MI, USA). The steroid hormones estrone, 17β-estradiol, testosterone and progesterone were measured by ELISA, also as per the manufacturer's instructions (ALPCO Immunoassays, Salem, NH, USA).
Spiking and recovery experiments were performed for progesterone and 17β-estradiol as representative ELISAs from the manufacturer to check for the recovery rates and applicability of coral WCL in these ELISA kits. Each sample was assessed in triplicate, spiked with pure steroid standard (manufacturer supplied) and recovery measured by comparison to the standard curve. The ELISA methodology above employs the use of antibodies specific to the chemical structure of interest, with less than 6% cross reactivity to structurally related chemicals (with the exception of the progesterone ELISA which cross reacts 100% with 11α-OH-Progesterone). Since, by definition, no two chemicals can have the same structure, the antibodies raised to pure chemicals represent a highly specific method for detection with far more restricted cross reactivity, usually only to chemical modifications of the same structure.
Kinetic Enzyme Assays
All steroidogenic assays were performed in 5 mL glass tubes, unless otherwise specified, and subsequently aliquoted in triplicate into 96 well clear microplates. For clearance and regeneration enzymes assays, the postmitochondrial tissue fraction of protein, assay buffer and substrate were loaded into wells on microplates that were kept on ice. Microplates were pre-warmed inside a microplate reader (Spectra Max or Gemini XS, Molecular Devices, Sunnyvale, CA) at 37 °C before addition of the cofactors used to initiate the reaction. Fluorescent assays were performed in solid black, flat-bottomed plates and colorimetric assays in clear plates. When necessary, substrates were dissolved in solvents (DMSO and methanol), which were never more than 2% of the reaction volume; hence solvent carrier properties should not have affected enzyme activities (Williams et al., 2008). Microplates were read using either a Spectra Max 340 Plus or Gemini XS (Molecular Devices, Sunnyvale, CA). Linearity studies were performed using pooled coral samples (n = 5) for each steroidogenic enzyme assay to determine linear time of reaction, optimal protein concentration, and optimal substrate concentration. Individual enzyme activities were assessed as described (reported values indicate final concentrations). Assays employed substrates specific for the particular enzyme and isoforms in vertebrates.
3β-Hydroxysteroid dehydrogenase (3βHSD)
The 3βHSD enzyme is responsible for the catalysis of progestagens and androgens, including the conversion of pregnenolone to progesterone. The activity of 3βHSD was determined by measuring the conversion of pregnenolone to progesterone as previously described (Bauer and Bauer, 1989; Raunig et al., 2011). ELISA and values were converted to ng/min/mg of protein using a standard curve of progesterone (manufacturer supplied).
Cytochrome P450 17 dehydrogenase (CYP17)
The conversion of the progesterone's 17α-hydroxy pregnenolone and 17α-hydroxy progesterone to the androgens dehydroepiandrosterone and androstenedione respectively, which are precursors in the pathway of estrone production, is performed by CYP17. Activity of CYP17 was determined using a method adapted from Holtorff and Koch (Holtorff and Koch, 1940). Briefly, the postmitochondrial tissue fraction of coral protein (20 μg) in 0.1 M Tris-HCl buffer pH 7.4, containing 50 mM MgCl2 and 500 μM 17α hydroxypregnenolone was added to glass tubes on ice. Tubes were preincubated for 30 s at 37 °C prior to reaction initiation through the addition of 1 mM NADPH. Tubes were then incubated at 37 °C for 5 min. Reactions were terminated through the addition of an equal volume of 5 M KOH. An equal volume of 2% m-dinitrobenzene, in 95% ethanol, was added and the color allowed to develop for 2 min. This mixture was transferred to 1.5 mL microcentrifuge tubes and spun at 10,000 x g for 3 min to pellet precipitate, in an Eppendorf MiniSpin centrifuge (Hauppauge, NY, USA). Absorbance was determined at λ = 520 nm in triplicate wells and results transformed to nmole/min/mg of protein using a standard curve of DHEA (0-1 mM).
Aromatase
Aromatase is responsible for the conversion of the androgens androstenedione and testosterone to the estrogens estrone and estradiol respectively. Aromatase activity was determined by measuring the conversion of testosterone to 17β-estradiol as previously described (Lephart and Simpson, 1991) and used by us (Sugawara et al., 2012). Detection of 17β-estradiol was determined through ELISA and results converted to fg/min/mg of protein using a standard curve of 17β-estradiol (manufacturer supplied).
UDP-glucuronosyl transferase (UGT)
UGT, a Phase II enzyme, conjugates glucuronic acid, a large polar group, to endogenous and xenobiotic compounds to produce more hydrophilic metabolites for excretion. Total UGT activity was determined using the method of Collier et al. (Collier et al., 2000) as previously described (Rougee et al., 2014). Results were transformed to pmole/min/mg protein using a standard curve generated with 4-methylumbelliferone (4-MU).
Sulfotransferase (SULT)
SULT, another Phase II enzyme, conjugates the addition of a sulfonate moiety to facilitate excretion of compounds. The activity of SULT1A1 was measured using the method of Frame et al. (Frame et al., 2000), while general SULT activity (including 1A1, 1A2, 1A3, 1B1, 1E1, 2A1 isoforms) was measured using the method by Tabrett and Coughtrie (Tabrett and Coughtrie, 2003).
β-Glucuronidase
β-glucuronidase catalyzes the hydrolysis of β-D-glucuronic acid residues on molecules returning them to more lipophilic forms, and helping prolong their duration. Activity for this enzyme was determined using the method of Trubetskoy and Shaw (Trubetskoy and Shaw, 1999) as previously described (Rougee et al., 2014). Fluorescence was continuously monitored at 355 nm ex/460 nm em. Results were transformed to pmole/min/mg protein using a standard curve generated with 4-MU.
Arylsulfatase C (ASC)
Arylsulfatase C, also referred to as steroid sulfatase, is responsible for converting sulfated steroids to their free steroid form. Activity of ASC was determined using a modification of the method of Roy (Roy, 1958) as previously described (Rougee et al., 2014). Results generated using a standard curve of paranitrophenol.
Statistical Analysis
Statistical analysis was performed using the GraphPad Prism Program version 5.02. Due to the small sample size, normality of the data was checked through the Kolmogorov-Smirnov test that employs a Dallal and Wilkinson approximation to the Lilliefors' method. Equality of variance of the samples was verified using the Bartlett's test. A one-way analysis of variance (ANOVA) was used if the data were found to be normally distributed and homogeneous. The Bonferroni's multiple comparison post hoc test was then used to compare differences between sample dates. However, if the data did not meet the requirements for normal distribution, the Kruskal-Wallis (KW) one-way ANOVA on ranks was performed to compensate, and the Dunn's post hoc test was employed to compare differences between sample dates. If significant differences in variance based on the Bartlett's test were found, the data was log transformed and a one-way ANOVA was performed. If the Bartlett's test on the transformed data was significant, the variances were considered unequal and a KW ANOVA was performed; otherwise, the one-way ANOVA was reported. If an ANOVA was performed, the p value of the original Bartlett's test was still reported and the variance described. Statistical significance was defined as p ≤ 0.05.
Results
Steroid Hormone Levels
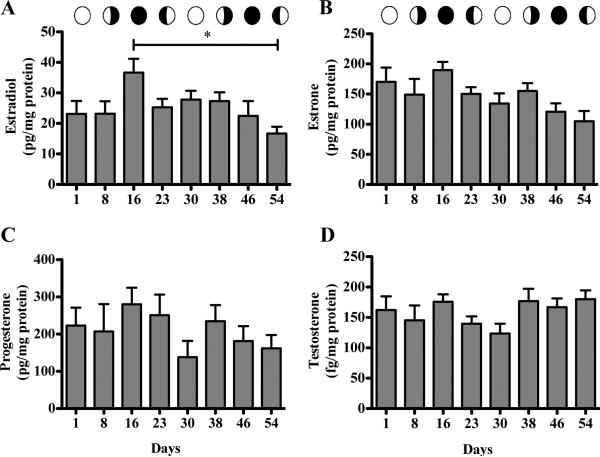
No significant differences were detected between any time points for total or free cholesterol (Figure 1). The same trend was observed for all steroid hormones, with the exception of 17β-estradiol (Figure 2). Although the ANOVA was not significant (p = 0.0540), the post hoc test detected a significant difference (p<0.05) between day16 and 54 of sampling (one week and two weeks after planulation time points respectively; Figure 2A). The average concentration range among the steroid hormones varied, with progesterone and estrone levels being 6-fold greater than those for 17β-estradiol (Table 1). Values for day 16 (one week after planulation) were consistently on the higher end of the range, while for day 30 and 54 (one week before and two week after planulation respectively) were on the lower end of the range. Results measured with coral WCL samples for progesterone and 17β-estradiol indicated that recovery rates were greater than 100% (due to residual steroid levels in the samples) and consistent (Intra-day CV = 6 and 7 % respectively).
Figure 1.
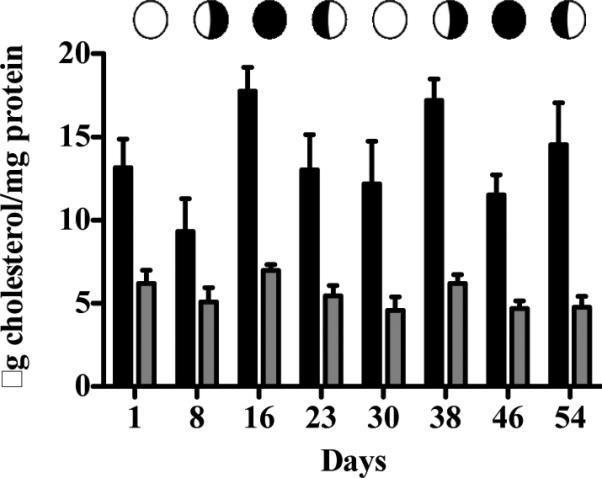
Cholesterol levels in Pocillopora damicornis. Total ( ) and free (
) and free ( ) levels over two consecutive lunar cycles (ANOVA). Bars are geometric means ± SEM for n = 5 determinations assayed in triplicate.
) levels over two consecutive lunar cycles (ANOVA). Bars are geometric means ± SEM for n = 5 determinations assayed in triplicate.
Figure 2.
Steroid hormones levels in Pocillopora damicornis A, 17β-Estradiol (ANOVA). B, Estrone (KW ANOVA). C, Progesterone (ANOVA). D, Testosterone (KW ANOVA). Capped line represents significant difference between time points at caps (Bonferroni p ≤ 0.05). Bars are geometric means ± SEM for n = 5 determinations assayed in triplicate.
Table 1.
Cholesterol and steroid hormone inter-colony concentrations. Numbers represent geometric mean ± SEM.
| Minimum | Maximum | Difference | Units | |
|---|---|---|---|---|
| Total Cholesterol | 9.32 ± 1.98 | 17.76 ± 1.43 | ~1.9 fold | μg cholesterol/mg protein |
| Free Cholesterol | 4.57 ± 0.82 | 6.97 ± 0.36 | ~1.5 fold | μg cholesterol/mg protein |
| Estrone | 104.9 ± 17.14 | 189.7 ± 13.63 | ~1.8 fold | pg/mg protein |
| 17β-Estradiol | 16.69 ± 2.26 | 36.66 ± 4.53 | ~2.2 fold | pg/mg protein |
| Progesterone | 137.8 ± 43.72 | 280.3 ± 44.41 | ~2.0 fold | pg/mg protein |
| Testosterone | 123.43 ± 36.54 | 180.05 ± 32.78 | ~1.5 fold | fg/mg protein |
Steroidogenic Enzymes
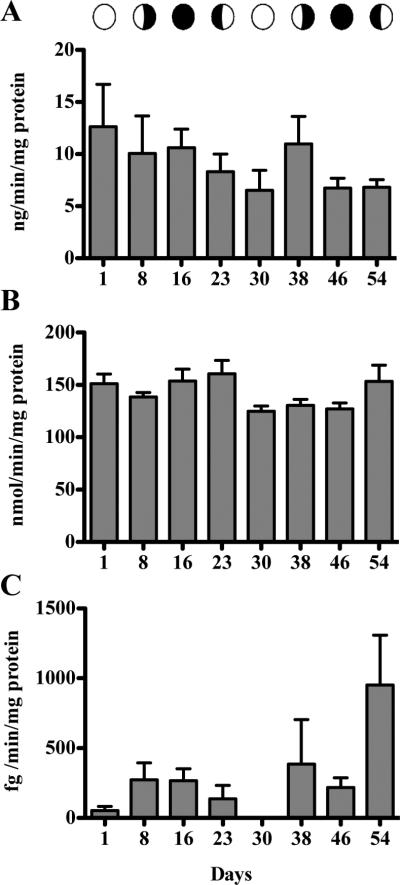
Activity of steroidogenic enzymes remained consistent during the coral reproductive cycle with no statistically significant differences observed. Activity of 3βHSD (Figure 3A) ranged from 6.78 ± 3.13 (Day 30) to 9.36 ± 2.4 (Day 16) ng/min/mg protein, while CYP 17 (Figure 3B) ranged from 125.0 ± 11.11 (Day 30) to 160.7 ± 28.46 (Day 23) nmole/min/mg protein. However, the variances for 3βHSD over the lunar cycle differed significantly (Bartlett's Test; p = 0.0329 prior to data transformation). This spread of activity at particular time points may indicate alterations in the induction and regulation of the enzyme. Aromatase activity was low, with several samples having non-detectable activity up to 951.3 ± 714.3 fg/min/mg protein (Figure 3C). No significant differences could be calculated for aromatase.
Figure 3.
Steroidogenic enzymes activities. A, 3βHSD (ANOVA). B, CYP17 (ANOVA). C, Aromatase (KW ANOVA). Bars are means ± SEM for n = 5 determinations assayed in triplicate.
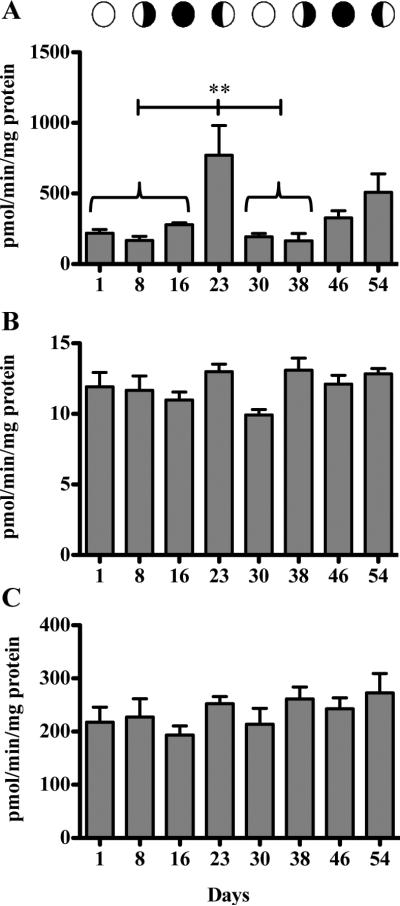
Steroid Clearance and Regeneration Enzymes
The sulfotransferases were not detected in either assay attempted for any coral sample, although positive controls showed that the assays worked. Activity of UGT enzymes was significantly higher (ANOVA p < 0.0001; post hoc p<0.05) at the two week after planulation time point for the first month of sampling (Day 23) at 770.8 ± 470.9 pmole/min/mg protein (Figure 4A). However, despite a general trend of increased activity observed after planulation (1 and 2 weeks after planulation), no significant differences were recorded for the second month. Additionally, variance between time points differed significantly (Bartlett's p<0.0001prior to data transformation) suggesting that regulation of the UGT enzyme activity may be altered relative to the lunar reproductive cycle. Reverse clearance enzymes β-glucuronidase and ASC did not fluctuate significantly over the lunar cycle (Figure 4C,D).
Figure 4.
Fluctuation of steroid clearance and regeneration enzymes over two reproductive lunar cycles. A, UGT (ANOVA p < 0.0001, Bonferroni p ≤ 0.01). B, β-glucuronidase (ANOVA). C, ASC (ANOVA). Capped line represents significant difference between time points under braces and day 23. Bars are means ± SEM for n = 5 determinations assayed in triplicate.
Discussion
Herein, we present evidence for the presence of the steroid hormones 17β-estradiol, estrone, progesterone, testosterone, as well as activity of steroidogenic enzymes 3βHSD, CYP17 and aromatase, in the brooding coral Pocillopora damicornis. Previous research efforts investigating the molecular mechanisms of coral reproduction have focused on coral species that reproduce through mass spawning events (Armoza-Zvuloni et al., 2012; Blomquist et al., 2006; Slattery et al., 1999; Slattery et al., 1997; Tarrant et al., 1999; Tarrant et al., 2003; Twan et al., 2006), with limited investigations conducted on brooding species (Gassman, 1992; Slattery et al., 1997; Tarrant et al., 1999; Tarrant et al., 2003). The unique physiology of P. damicornis, a hermaphroditic brooder that planulates every month of the year, makes this species an ideal model for studying the molecular mechanisms and responses of proteins involved in coral reproduction.
Our study detected no significant fluctuations (with minor exceptions) for steroid hormone precursors, steroid hormones or steroidogenic enzymes, over the corals’ lunar reproductive cycle in P. damicornis. Assuming that steroid hormones play a role in coral reproductive physiology, these results align with previous observations of oocyte maturation in P. damicornis (Stoddart and Black, 1985). Histological investigations have recorded the presence of multiple stages of maturing oocytes in addition to the developing planulae in P. damicornis (Permata et al., 2000; Stoddart and Black, 1985). Thus, as each cohort of planulae is being prepared for release, the following two months’ brood is developing. This contrasts with, for example; the human oocyte, where sex steroids peak and subside in a series of hormonal events to drive oocyte maturation and release in the follicular and luteal phases (Porterfield and White, 2007). Therefore, for P. damicornis, sustained high levels of steroid hormones are probably necessary for continuous gametogenesis, embryo maturation and development, and could account for the lack of fluctuations in the levels of steroid hormones measured in this study. However, since the true role of steroid hormones in corals has not been elucidated, further investigations are needed to determine the significance of these findings.
One observation of particular interest is the fluctuation in clearance enzyme activity during the corals lunar reproductive cycle. While no significant changes were observed for the regeneration enzymes β-glucuronidase or ASC, the clearance enzyme UGT fluctuated significantly over the lunar cycle, peaking two weeks post-planulation. The results from the current study over two consecutive lunar cycles, confirm previous findings in our laboratory, and suggest that the regulation of this enzyme is linked to the reproductive cycle of the coral (Rougee et al., 2014). The UGT superfamily is variously responsible for glucuronidation of toxicants (Bock and Schirmer, 1987; Jin et al., 1993; Orzechowski et al., 1994) as well as endogenous ligands and steroid hormones (Bélanger et al., 1998; Hum et al., 1999). Regulation of this pathway can play a role in the predisposition to toxicity. Our results suggest that P. damicornis may be more susceptible and/or sensitive to toxicants, regulated through glucuronidation, if exposed at time points between planulation events when UGT activity is decreased. This brings attention to “point-intime” studies. Since enzymes can serve multiple roles outside of detoxification, characterization of the natural fluctuations and baseline levels are paramount to understanding how environmental pollutants can disrupt the pathways at specific times during the coral life cycle.
A comparison of the maximal enzyme activity rates between excretion and reverse cleavage shows that the system favors excretion through glucuronidation, while in the sulfation pathway, retention of compounds is favored. However, these statements are tempered by our inability to detect SULT enzymes in coral. Analysis of the Nematostella vectensis genome discovered that the SULT genes present were more closely related to the membrane bound SULT enzymes involved in energy metabolism than to the cytosolic SULTs associated with detoxification reactions in vertebrates (Goldstone, 2008). Additionally, the substrates employed (4-nitrophenol and 2-naphthol) in our investigation may not be recognized by coral SULTs. These characteristics, along with our use of a spectrophotometric method as opposed to mass spectrometry, likely explain our lack of detection of SULT enzymes in coral. Investigation of SULT at the individual isoform level is needed to elucidate the existence of sulfotransferase pathways in corals.
The timing and control of the molecular reproductive axes in corals still remain unclear. Steroids and their conjugated forms have been detected consistently within coral tissue and in surrounding seawater, with altered levels centered around reproductive events, suggesting the release of steroids into the surrounding water as a potential cue for spawning synchronicity (Armoza-Zvuloni et al., 2012; Blomquist et al., 2006; Pernet and Anctil, 2002; Slattery et al., 1999; Slattery et al., 1997; Tarrant et al., 1999; Tarrant et al., 2003; Twan et al., 2003; Twan et al., 2006). Additionally, corals have been shown to uptake estrogens dissolved in seawater, resulting in a negative feedback on coral reproductive capacity and decreased skeletal growth rate (Tarrant et al., 2001; Tarrant et al., 2004). However, while corals have the capacity to inter-convert steroid hormones (e.g. convert estradiol to estrone) and contain steroid hormones, to date, no evidence exists to suggest that corals produce steroid hormones de novo (Tarrant et al., 1999; Tarrant et al., 2003; Twan et al., 2006). The contribution of the current study is that P. damicornis has the precursor to steroid hormones (cholesterol). Combining the evidence herein with evidence from other studies regarding enzymes responsible for the production and conversion of steroid hormones, it is suggested that corals have the necessary tools capable of synthesizing steroid hormones de novo within their tissues.
Although many examples of reduced coral reproductive capacity, reproductive failure, and continuous reef decline have been documented (Hughes and Tanner, 2000; Knowlton, 2001; Szmant, 2002), definitive answers linking the cause to the effect are lacking. General observations have provided a wealth of descriptive information on coral reproduction. However, coral communication for synchronous reproduction and molecular pathways for production of gametes (sperm and egg) as well as the mechanisms for disrupting these natural events remain unknown. Therefore, understanding the magnitude and range of effects of “endocrine disruptors” cannot be advanced without a detailed understanding of molecular mechanisms for reproduction and signaling in these species, as well as changes in nonreproductive pathways that may fluctuate with the reproductive cycle. Here we present the first step towards defining normal physiological and lunar/reproductive variability in a coral species. Further understanding of coral reproduction at the molecular level will be necessary to completely understand its disruption, which is of great concern for environmental and marine pollutants, and the persistence of coral reefs as a whole.
Acknowledgements
We thank the Hawaii Human Organ and Tissue Bank and staff of the Organ Donor Center of Hawaii for providing the human tissues used as positive controls in these experiments. Use of these archival tissues, from deceased donors, is considered exempt by The University of Hawaii Institutional Review Board for Human Subjects. Partial funding support was provided by grant NA09NOS4780178 from NOAA/NCCOS/CSCOR and the Pew Environmental Group to R. H. Richmond.
Funding: This research was supported by grants to Dr. Robert Richmond from The Pew Environmental Group, NOAA grant (NA09NOS4780178) from the Center for Sponsored Coastal Ocean Research and the Hawaii Coral Reef Initiative, and from the National Institute of Health RCMI/BRIDGES (G12MD007601) Core 3 (Dr. Abby C Collier, Director).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement: The authors have nothing to disclose.
References
- Armoza-Zvuloni R, Kramarsky-Winter E, Rosenfeld H, Shore LS, Segal R, Sharon D, Loya Y. Reproductive characteristics and steroid levels in the scleractinian coral Oculina patagonica inhabiting contaminated sites along the Israeli Mediterranean coast. Mar. Pollut. Bull. 2012;64:1556–1563. doi: 10.1016/j.marpolbul.2012.05.020. [DOI] [PubMed] [Google Scholar]
- Bauer HC, Bauer H. Micromethod for the determination of 3-beta-HSD activity in cultured cells. J. Steroid Biochem. 1989;33:643–646. doi: 10.1016/0022-4731(89)90054-x. [DOI] [PubMed] [Google Scholar]
- Bélanger A, Hum DW, Beaulieu M, Lévesque E, Guillemette C, Tchernof A, Bélanger G, Turgeon D, Dubois S. Characterization and regulation of UDP-glucuronosyltransferases in steroid target tissues. J. Steroid Biochem. Mol. Biol. 1998;65:301–310. doi: 10.1016/s0960-0760(97)00183-0. [DOI] [PubMed] [Google Scholar]
- Blomquist CH, Lima PH, Tarrant AM, Atkinson MJ, Atkinson S. 17Beta-hydroxysteroid dehydrogenase (17beta-HSD) in scleractinian corals and zooxanthellae. Comp. Biochem. Physiol. B. Biochem. Mol. Biol. 2006;143:397–403. doi: 10.1016/j.cbpb.2005.12.017. [DOI] [PubMed] [Google Scholar]
- Bock KW, Schirmer G. Species differences of glucuronidation and sulfation in relation to hepatocarcinogenesis. Archives of toxicology. Supplement. = Archiv fur Toxikologie. 1987;(Supplement 10):125–135. doi: 10.1007/978-3-642-71617-1_10. [DOI] [PubMed] [Google Scholar]
- Cesar H, Burke L, Pet-Soede L. The economics of worldwide coral reef degredation, Cesar Environmental Economics Consulting. Arnhem,The Netherlands. 2003:23. [Google Scholar]
- Collier AC, Tingle MD, Keelan JA, Paxton JW, Mitchell MD. A highly sensitive fluorescent microplate method for the determination of UDP-glucuronosyl transferase activity in tissues and placental cell lines. Drug Metab. Disposition. 2000;28:1184–1186. [PubMed] [Google Scholar]
- Evans RM. The steroid and thyroid hormone receptor superfamily. Science. 1988;240:889–895. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frame LT, Ozawa S, Nowell SA, Chou HC, DeLongchamp RR, Doerge DR, Lang NP, Kadlubar FF. A simple colorimetric assay for phenotyping the major human thermostable phenol sulfotransferase (SULT1A1) using platelet cytosols. Drug Metab. Disposition. 2000;28:1063–1068. [PubMed] [Google Scholar]
- Gassman NJ. Aspects of the steroid biochemistry of the scleractinian coral, Favia fragum, Marine Biology and Fisheries. Vol. 158. University of Miami, Dissertations from ProQuest; 1992. [Google Scholar]
- Goldstone JV. Environmental sensing and response genes in cnidaria: the chemical defensome in the sea anemone Nematostella vectensis. Cell Biol. Toxicol. 2008;24:483–502. doi: 10.1007/s10565-008-9107-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorski J, Gannon F. Current models of steroid hormone action: a critique. Annu. Rev. Physiol. 1976;38:425–450. doi: 10.1146/annurev.ph.38.030176.002233. [DOI] [PubMed] [Google Scholar]
- Holtorff AF, Koch FC. The colorimetric estimation of 17-ketosterois and their application to urine extracts. J. Biol. Chem. 1940;135:377–392. [Google Scholar]
- Hughes TP, Tanner JE. Recruitment failure, life histories and long-term decline of caribbean corals. Ecology. 2000;81:2250–2263. [Google Scholar]
- Hum DW, Bélanger A, Lévesque E, Barbier O, Beaulieu M, Albert C, Vallée M, Guillemette C, Tchernof A, Turgeon D, Dubois S. Characterization of UDP-glucuronosyltransferases active on steroid hormones. J. Steroid Biochem. Mol. Biol. 1999;69:413–423. doi: 10.1016/s0960-0760(99)00061-8. [DOI] [PubMed] [Google Scholar]
- Jin CJ, Miners JO, Lillywhite KJ, Mackenzie PI. cDNA cloning and expression of two new members of the human liver UDP-glucuronosyltransferase 2B subfamily. Biochem. Biophys. Res. Commun. 1993;194:496–503. doi: 10.1006/bbrc.1993.1847. [DOI] [PubMed] [Google Scholar]
- Johannes RE, Wiebe WJ. Method for determination of coral tissue biomass and composition. Limnol. Oceanogr. 1970;15:822–824. [Google Scholar]
- Knowlton N. The future of coral reefs. Proceedings of the National Academy of Sciences. 2001;98:5419–5425. doi: 10.1073/pnas.091092998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolinski SP, Cox EF. An update on modes and timing of gamete and planula release in Hawaiian Scleractinian corals with implications for conservation and management. Pac. Sci. 2003;57:17–27. [Google Scholar]
- Lephart ED, Simpson ER. Assay of aromatase activity. Methods Enzymol. 1991;206:477–483. doi: 10.1016/0076-6879(91)06116-k. [DOI] [PubMed] [Google Scholar]
- Orzechowski A, Schrenk D, Bock-Hennig BS, Bock KW. Glucuronidation of carcinogenic arylamines and their N-hydroxy derivatives by rat and human phenol UDP-glucuronosyltransferase of the UGT1 gene complex. Carcinogenesis. 1994;15:1549–1553. doi: 10.1093/carcin/15.8.1549. [DOI] [PubMed] [Google Scholar]
- Permata WD, Kinzie RA, III, Hidaka M. Histological studies on the origin of planulae of the coral Pocillopora damicornis. Mar. Ecol. Prog. Ser. 2000;200:191–200. [Google Scholar]
- Pernet V, Anctil M. Annual variations and sex-related differences of estradiol-17beta levels in the anthozoan Renilla koellikeri. Gen. Comp. Endocrinol. 2002;129:63–68. doi: 10.1016/s0016-6480(02)00513-0. [DOI] [PubMed] [Google Scholar]
- Porterfield SP, White BA. Endocrine physiology. 3rd ed. Mosby/Elsevier; Philadelphia, PA.: 2007. [Google Scholar]
- Raunig JM, Yamauchi Y, Ward MA, Collier AC. Assisted reproduction technologies alter steroid delivery to the mouse fetus during pregnancy. J. Steroid Biochem. Mol. Biol. 2011;126:26–34. doi: 10.1016/j.jsbmb.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond RH, Hunter CL. Reproduction and recruitment of corals: comparisons among the Caribbean, the Tropical Pacific, and the Red Sea. Mar. Ecol. Prog. Ser. 1990;60:185–203. [Google Scholar]
- Richmond RH, Jokiel PL. Lunar periodicity in larva release in the reef coral Pocillopora damicornis at Enewetak and Hawaii. Bull. Mar. Sci. 1984;34:280–287. [Google Scholar]
- Rougee LRA, Richmond RH, Collier AC. Natural variations in xenobiotic metabolizing enzymes: Developing tools for coral monitoring. Coral Reefs. 2014 In Press. [Google Scholar]
- Roy AB. Comparative studies on the liver sulphatases. Biochem. J. 1958;68:519–528. doi: 10.1042/bj0680519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slattery M, Hines GA, Starmer J, Paul VJ. Chemical signals in gametogenesis, spawning, and larval settlement and defense of the soft coral Sinularia polydactyla. Coral Reefs. 1999;18:75–84. [Google Scholar]
- Slattery M, Hines GA, Watts SA. Steroid metabolism in Antarctic soft corals. Polar Biol. 1997;18:76–82. [Google Scholar]
- Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Stoddart JA, Black R. Cycles of gametogenesis and planulation in the coral Pocillopora damicornis. Mar. Ecol. Prog. Ser. 1985;23:153–164. [Google Scholar]
- Sugawara A, Sato B, Bal E, Collier AC, Ward MA. Blastomere removal from cleavage-stage mouse embryos alters steroid metabolism during pregnancy. Biol. Reprod. 2012;87:4, 1–9. doi: 10.1095/biolreprod.111.097444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szmant AM. Nutrient enrichment on coral reefs: is it a major cause of coral reef decline? Estuaries. 2002;25:743–766. [Google Scholar]
- Tabrett CA, Coughtrie MW. Phenol sulfotransferase 1A1 activity in human liver: kinetic properties, interindividual variation and re-evaluation of the suitability of 4-nitrophenol as a probe substrate. Biochem. Pharmacol. 2003;66:2089–2097. doi: 10.1016/s0006-2952(03)00582-3. [DOI] [PubMed] [Google Scholar]
- Tarrant AM, Atkinson MJ, Atkinson S. Uptake of estrone from the water column by a coral community. Mar. Biol. 2001;139:321–325. [Google Scholar]
- Tarrant AM, Atkinson MJ, Atkinson S. Effects of steroidal estrogens on coral growth and reproduction. Mar. Ecol. Prog. Ser. 2004;269:121–129. [Google Scholar]
- Tarrant AM, Atkinson S, Atkinson MJ. Estrone and estradiol-17 beta concentration in tissue of the scleractinian coral, Montipora verrucosa. Comp. Biochem. Physiol. A. Mol. Integr. Physiol. 1999;122:85–92. doi: 10.1016/s1095-6433(98)10155-1. [DOI] [PubMed] [Google Scholar]
- Tarrant AM, Blomquist CH, Lima PH, Atkinson MJ, Atkinson S. Metabolism of estrogens and androgens by scleractinian corals. Comp. Biochem. Physiol. B. Biochem. Mol. Biol. 2003;136:473–485. doi: 10.1016/s1096-4959(03)00253-7. [DOI] [PubMed] [Google Scholar]
- Trubetskoy OV, Shaw PM. A fluorescent assay amenable to measuring production of beta-D-glucuronides produced from recombinant UDP-glycosyl transferase enzymes. Drug Metab. Disposition. 1999;27:555–557. [PubMed] [Google Scholar]
- Twan WH, Hwang JS, Chang CF. Sex steroids in scleractinian coral, Euphyllia ancora: implication in mass spawning. Biol. Reprod. 2003;68:2255–2260. doi: 10.1095/biolreprod.102.012450. [DOI] [PubMed] [Google Scholar]
- Twan WH, Hwang JS, Lee YH, Wu HF, Tung YH, Chang CF. Hormones and reproduction in scleractinian corals. Comp. Biochem. Physiol. A. Mol. Integr. Physiol. 2006;144:247–253. doi: 10.1016/j.cbpa.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Veron JEN. Corals in space & time. The biogeography and evolution of the scleractinia. Cornell University Press; Ithaca, NY.: 1995. [Google Scholar]
- Veron JEN. Corals of the world. Australian Institute of Marine Science; Townsland, QLD.: 2000. [Google Scholar]
- Williams ET, Ehsani ME, Wang X, Wang H, Qian YW, Wrighton SA, Perkins EJ. Effect of buffer components and carrier solvents on in vitro activity of recombinant human carboxylesterases. J. Pharmacol. Toxicol. Methods. 2008;57:138–144. doi: 10.1016/j.vascn.2007.11.003. [DOI] [PubMed] [Google Scholar]