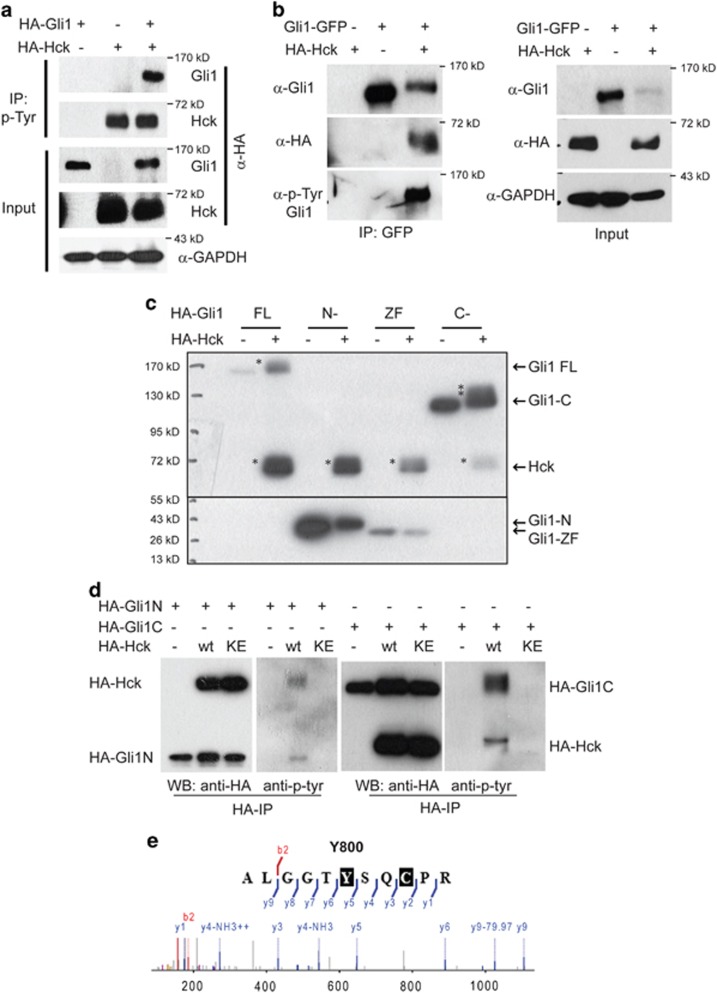
Figure 4.
Hck phosphorylates multiple residues in Gli1 through direct binding. (a) Gli1 is Tyr phosphorylated in the presence of exogenous Hck. NIH3T3 cells were transiently transfected with constructs expressing HA-tagged Gli1 alone, Hck alone or the two together. Cell lysates were immunoprecipitated using 4G10 antibody, followed by western blot using antibody against HA tag. (b) Gli1 co-precipitates with Hck. NIH3T3 cells were transfected with constructs for expression of GFP-Gli1, HA-Hck or the two together. Antibodies against GFP were used for the immunoprecipitation and antibodies against Gli1, HA and phospho-Tyr (4G10) were used for western blot. HA-Hck was co-precipitated with GFP-Gli1. Gli1 was Tyr phosphorylated in the presence of Hck as shown by the 4G10-positive band. GAPDH was used as a loading control. (c) Western blot analyses of Hck phosphorylated human Gli1 fragments (FL, full-length; N-, N-terminal fragment (1-231 aa); ZF, zinc-finger domain (232-410 aa); C-, C-terminal fragment (411-1106 aa)) separated on an SDS–PAGE gel. NIH3T3 cells were co-transfected with constructs for expression of Gli1 fragments and HA-Hck. Asterisks indicate the phosphorylated bands; arrows point to the corresponding non-phosphorylated protein bands. (d) Both the N- and C-terminal fragments of Gli1 were Tyr phosphorylated in the presence of Hck. NIH3T3 cells were transfected with constructs for expression of HA-tagged Gli1 fragments and HA-Hck or the kinase-inactive Hck-K269E. Antibodies against HA were used for the immunoprecipitation and antibodies against HA, and phospho-Tyr (4G10) were used for western blot. (e) HPLC-MS/MS spectrum of phosphopeptide ALGGTY(p)SQCPR that contains Y800. The ion peak labeled with minus 79.97 (H3PO4 mass) serves to confirm the Y800 phosphorylation.