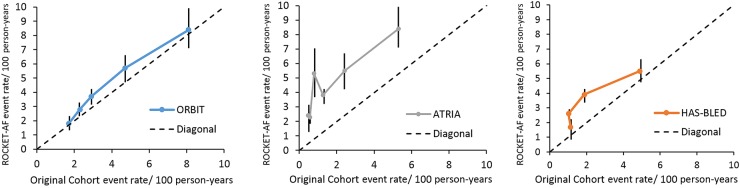
Figure 1.
Calibration plot of outcomes registry for better informed treatment, hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, labile INR, elderly, drugs/alcohol concomitantly, and anticoagulation and risk factors in atrial fibrillation in the rivaroxaban once-daily oral direct factor Xa inhibition compared with vitamin K antagonism for prevention of stroke and embolism trial in atrial fibrillation external validation cohort. This figure displays the major bleeding events rates per 100 patient-years and 95% confidence intervals observed in the external validation rivaroxaban once-daily oral direct factor Xa inhibition compared with vitamin K antagonism for prevention of stroke and embolism trial in atrial fibrillation cohort vs. those previously published from the original derivation cohorts for each discrete score point value. The highest risk categories for each score were combined to promote stable estimates as follows: outcomes registry for better informed treatment (0, 1, 2, 3, ≥4), anticoagulation and risk factors in atrial fibrillation (0, 1, 2, 3, ≥4), and hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, labile INR, elderly, drugs/alcohol concomitantly (0, 1, 2, ≥3). ORBIT-AF; Outcomes Registry for Better Informed Treatment of Atrial Fibrillation; ROCKET-AF, Rivaroxaban Once-daily oral direct factor Xa inhibition compared with vitamin K antagonism for prevention of stroke and embolism trial in atrial fibrillation; ATRIA, anticoagulation and risk factors in atrial fibrillation.