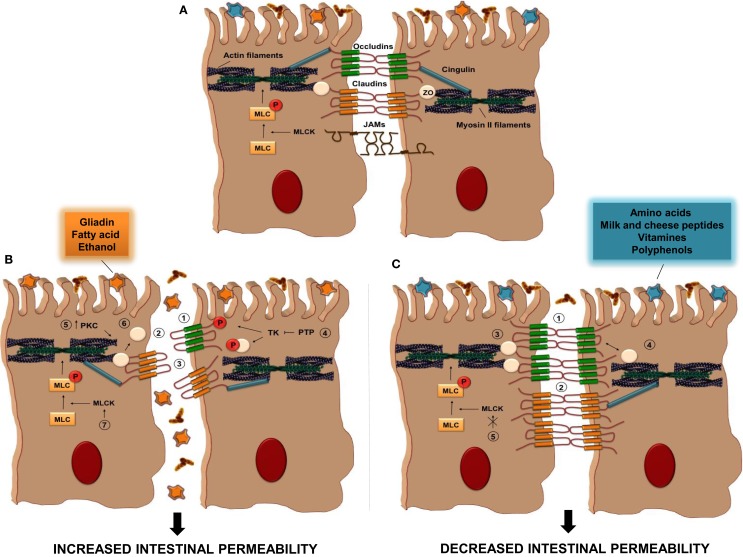
Figure 2.
Tight Junction regulation by food antigens. (A) TJs are composed of some transmembrane proteins [occludin, claudins, and junctional adhesion molecules (JAMs)] and cytosolic scaffold proteins [zonulae occludens (ZO) and cingulin]. The extracellular domains of transmembrane proteins of adjacent IECs interact to form the selective intestinal barrier, while cytosolic scaffolds anchor the transmembrane proteins to the actomyosin ring. (B) The intake of some food antigens, such as gliadin, fatty acids, or ethanol, can directly increase intestinal permeability by different mechanisms (1–7); (1) alteration in cellular distribution of occludin proteins, (2) reduction in the cellular content of occludins, (3) alteration in cellular distribution of claudin, (4) inhibition of protein tyrosine phosphatase (PTP) activity that induce tyrosine phosphorylation of ZO-1 and occludin and their dissociation from the junctional complex, (5) activation of PKC that leads to polymerization of actin and subsequent displacement of TJ proteins, including ZO-1, (6) displacement of ZO proteins from the junctional complexes, (7) activation of MLCK activity. (C) Other dietary antigens, such as amino acid, milk and cheese peptides, vitamins, and polyphenols, have the ability to decrease intestinal permeability through distinct pathways; (1–3) increase in the cellular content of occludin, claudin, and ZO proteins, respectively, (4) restoration of ZO-1/occludin assembly, (5) inhibition of MLCK activation. PKC, protein kinase C; MLC, myosin light-chain; MLCK, myosin light-chain kinase; TK, tyrosine kinase.