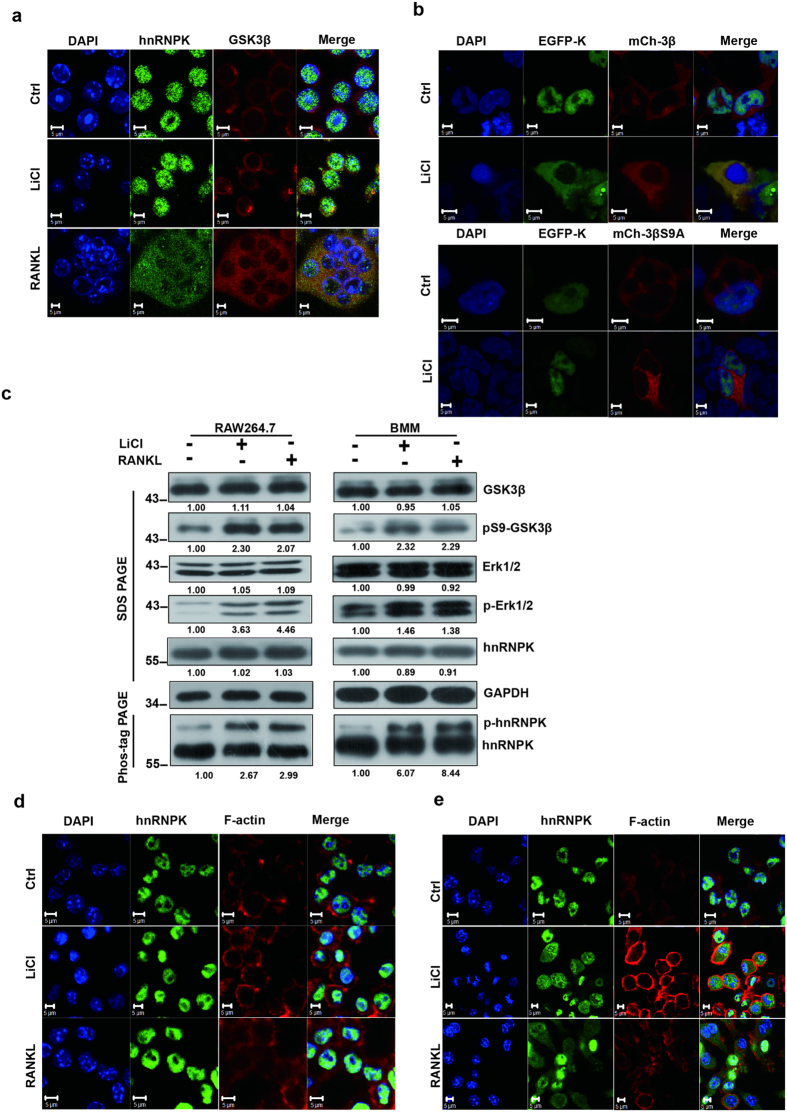
Figure 4. RANKL or LiCl induces the nuclear-cytoplasmic translocation of hnRNPK and its co-localization with GSK3β.
(a) The subcellular distribution of hnRNPK and GSK3β by confocal immunofluorescence experiments with RAW264.7 cells treated with 10 mM LiCl during 12 hours, or with 100 ng RANKL during 72 hours. hnRNPK was stained in green and GSK3β in red. (b) HEK293T cells were co-transfected with EGFP-hnRNPK (EGFP-K) and mCherry-GSK3β wt (mCh-3β, upper panels) or mCherry-GSK3β S9A (mCh-3βS9, lower panels) constructs. The subcellular localization of these fusion proteins were then examined under confocal microscopy. Scale bars = 5 μM. (c) RAW264.7 or BMM cells were treated either with 10 mM LiCL or 100 ng/mL RANKL during 12 hours, the protein extract of these cells were subjected to Western blotting to assess the total and phosphorylated levels for ERK or GSK3β, the phosphorylated and unphosphorylated levels of hnRNPK using appropriate antibodies. The numbers under each protein band indicate the fold changes after normalization using GAPDH and in comparison with the control group. For hnRNPK, only the quantitation result of the phosphorylated form was presented. Gels were run under the same experimental conditions except for the Phos-tag-PAGE. For better clarity and conciseness of the presentation, cropped blots are shown. The raw uncropped images can be found in the Supplementary Figure 1. The cropping lines are indicated by black lines both in cropped and uncropped images. The results shown are representative of at least 3 experiments. (d) RAW264.7 cells were co-treated by either 100 ng/mL RANKL for 48 hours or 10 mM LiCl for 12 hours with 20 μM u0126, and subjected to immunofluorescence assays to determine the subcellular localization of hnRNPK. (e) In parallel, control cells were co-treated by either 100 ng/mL RANKL for 48 hours or 10 mM LiCl for 12 hours with DMSO, and analyzed similarly as in (d). The results shown are representative of at least 3 experiments.