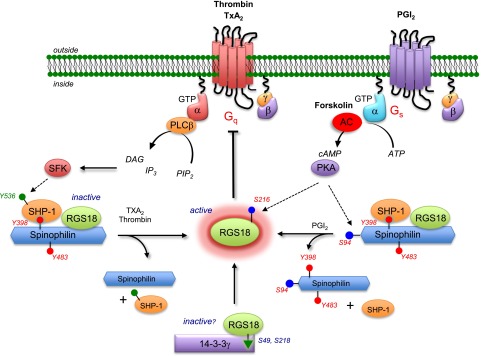
Figure 7.
A model for modulating platelet reactivity through regulated control of RGS proteins. In resting platelets, RGS18 is part of a complex that includes SPL and SHP-1 in which SPL is phosphorylated on tyrosines 398 and 483. Addition of thrombin or a TxA2 mimetic causes dissociation of the complex and dephosphorylation of SPL, freeing RGS18. Some of the SPL binds to PP1 once the SPL/RGS18/SHP-1 complex has dissociated (lower left). Addition of PGI2 or forskolin leads to cAMP-dependent phosphorylation of serine 94 in SPL, followed by dissociation of the SPL/RGS18/SHP-1 complex without dephosphorylation of SPL tyrosine residues (lower right). Both events cause an increase in free RGS18 available to bind to activated Gq and Gi, helping to limit platelet activation. Rising cAMP levels also result in phosphorylation of RGS18 Ser216, which Gegenbauer et al27 have shown to displace RGS18 from its binding site on 14-3-3γ (lower middle). AC, adenylyl cyclase; ATP, adenosine triphosphate; DAG, diacylglycerol; PIP, phosphatidylinositol 4,5-bisphosphate; PLC, phospholipase C; SFK, Src family kinases.