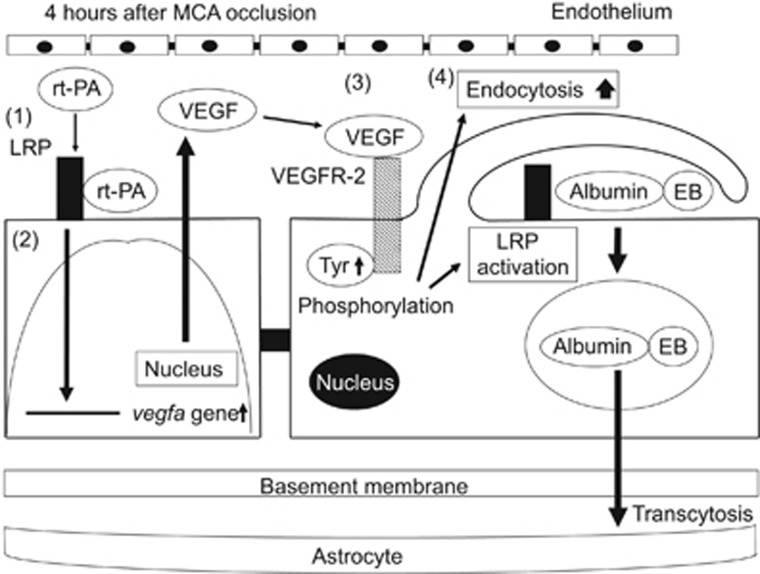
Figure 7.
Schematic mechanisms of the increase in blood–brain barrier permeability by recombinant tissue-type plasminogen activator treatment after ischemic stroke. Recombinant tissue-type plasminogen activator (rt-PA) activates low-density lipoprotein receptor-related protein (LRP), which is upregulated in endothelial cells by ischemic stress (1). The activation of LRP induces the enhanced accumulation of hypoxia-inducible factor-1 alpha (HIF-1α) in the nucleus. However, the transcriptional upregulation of vascular endothelial growth factor (VEGF) by LRP activation was not induced through the enhancement of HIF-1α accumulation (2). Secreted VEGF binds to VEGF receptor-2 (VEGFR-2) on the surface of endothelial cells through an autocrine mechanism and induces its phosphorylation (3). The activation of VEGFR-2 leads to an increase in endocytosis and to the activation of LRP, resulting in enhanced blood–brain barrier (BBB) permeability by endocytosis and subsequent transcellular transport of proteins into cerebroparenchyma (4).