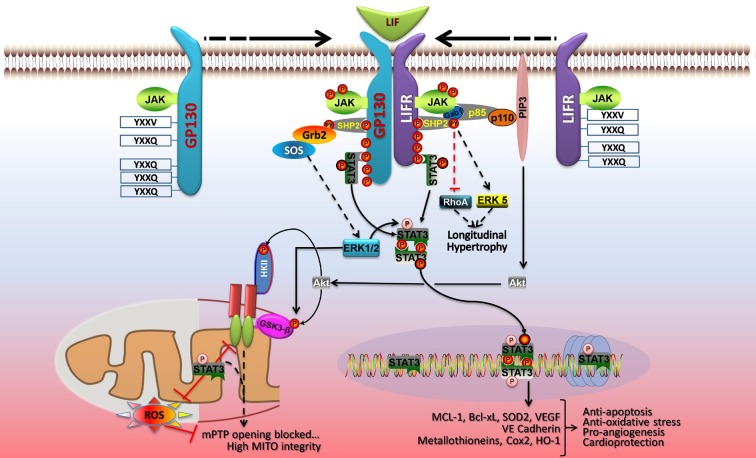
Figure 3.
LIF protects cardiac myocytes by both genomic and non-genomic means. Dimerization of gp130 and LIFR activates the associated JAK tyrosine kinases, which phosphorylate recruitment sites for STAT3 and STAT1 (YXXQ), and a scaffold protein SHP2 (YXXV) that is coupled to ERK1/2, ERK5, and phosphoinositide 3-kinase (PI3K)-Akt activation. STAT3 and the SHP2-based signaling pathways are linked to cellular protection and/or cell growth. JAK-mediated STAT3 phosphorylation on Y705 enhances its dimerization and DNA binding. Activated STAT3 induces expression of anti-apoptotic, anti-oxidative stress, and pro-angiogenic genes, and STAT3 S727 phosphorylation boosts gene transcription. In the nucleus, U-STAT3 may affect gene expression either as a transcription factor or by modulating chromatin structure. In parallel, STAT3 has protective actions on mitochondrial function. STAT3 optimizes mitochondrial respiration and limits ROS formation, thereby opposing mPTP opening and ensuring mitochondrial integrity. These non-genomic actions of STAT3 are enhanced by S727 phosphorylation. ERK1/2 and Akt are components of the RISK pathway of cardiac protection that is evoked by pre- and postconditioning. ERK1/2 has protective effects on mitochondria via phosphorylation and inhibition of GSK3-α/β, which prevents mPTP opening. PI3K-Akt signaling also inhibits opening of mPTP via phosphorylation of GSK3-α/β or mitochondrial hexokinase II (HKII). See text for additional details. Adapted with permission from Zouein et al. (62).