Abstract
Patient: Female, 68
Final Diagnosis: Gastrointestinal stromal tumour and colon adenocarcinoma
Symptoms: Fatigue
Medication: —
Clinical Procedure: Right Hemicolectomy and enterectomy
Specialty: Surgery
Objective:
Rare disease
Background:
GISTs are mesenchymal tumors representing approximately 1% of all gastrointestinal neoplasia. Their concurrence with colorectal cancers is rare.
Case Report:
We present a case of coexistence of colon adenocarcinoma and GIST of the ileum in a 68-year-old white woman.
Conclusions:
The coexistence of mesenchymal and epithelial neoplasia is very challenging; further research is needed to clarify the role of oncogenic mutations and signalling pathways in carcinogenesis of neoplasia of various histiogenic origins.
MeSH Keywords: Colectomy, Colonic Neoplasms, Gastrointestinal Stromal Tumors
Background
GISTs are the most common mesenchymal tumors of the GI tract and can appear from the oesophagus to the anus. They arise from the interstitial cells of Cajal [1]. The coexistence of GIST and colorectal adenocarcinomas is rare. Most cases of associated GIST and adenocarcinomas have been described in the stomach. In most cases GIST was discovered incidentally during an operation for primary gastrointestinal adenocarcinoma [1,2]. Studies based on the expression of the c-kit proto-oncogene support the hypothesis of common carcinogenic etiology. However, the few published cases cannot rule out a possible incidental occurrence of GIST and adenocarcinoma [3].
Case Report
A 68-year-old white woman was admitted with the endoscopic and pathologic diagnosis of adenocarcinoma of the ascending colon. Apart from low hemoglobin (Hb10.3), her past medical history was unremarkable. The preoperative CT scan revealed a colonic tumor of the ascending colon but did not detect any associated tumour elsewhere in the gastrointestinal tract. She underwent a right hemicolectomy and during the abdominal exploration an extramural tumor of the ileum (5.5×5.8×4.5 cm) was found (Figure 1). Subsequently, an enterectomy was performed and both specimens were sent to pathology. Pathology reported well-circumscribed GIST spindle cells infiltrating the submucosa, muscularis propria, and subserosal layers of the small bowel. There was neither mucosa invasion nor serosal breach. Mitotic activity was inconspicuous. Upon immunostaining, the tumor cells demonstrated strong diffuse positivity with CD117, BC22, SMA, and CD34. Focal positivity was seen with S 100. The tumor cells were negative for desmin and MNF116. On the basis of the sample received, according to Miettinen criteria for risk stratification, this tumor has a moderate risk (24%) of progressive disease (localized in the ileum, less than 5 mitoses in 5 mm2, >5 cm, and <10 cm in size). The patient recovered uneventfully and was discharged on the 5th postoperative day. She is scheduled for follow-up visits every 3 months in colorectal outpatient clinics.
Figure 1.
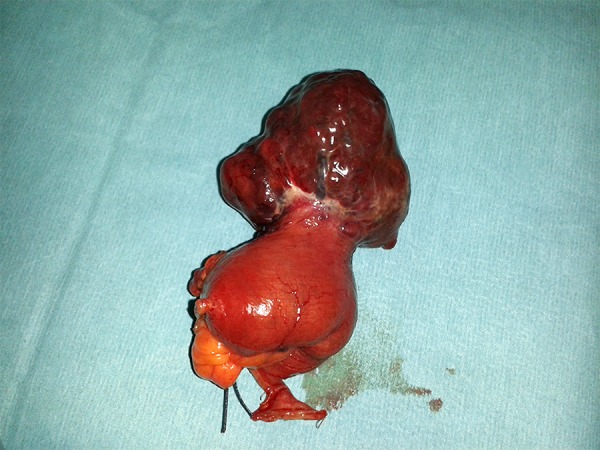
Typical extramural appearance of GIST of the small bowel.
Discussion
The term GIST was coined for the first time in 1983 to define neoplasms with complete lack of myogenic or neural component [4]. Somatic mutations trigger the process of carcinogenesis in most GISTs by activating the KIT signalling pathway [5]. Activated KIT subsequently phosphorylates JAK, STAT, MAP kinase, and PI3 kinases, which in turn activate signalling cascades that play vital roles in differentiation and mitogenesis of GIST neoplasms [5].
Mutations of KIT protein can be detected in 80% of benign and in 90% of metastatic GISTs [6]. PDGFR-a and BRAF mutations are alternative molecular pathways in GIST tumorigenesis, in particular BRAF mutations are related with benign intestinal GIST of low malignancy potential [7,8]. Studies on p16 tumor suppressor protein showed that patients with p16 loss have a dismal prognosis [9,10]. GISTs occur most often in the stomach (60%); intestine (30%); colon (<5%), mesentery, omen-tum, and retroperitoneum (< 5%); anorectum (<5%); and oesophagus (3%) [11]. Most GISTs appear sporadically and are occasionally identified in rare syndromes such as neurofibromatosis type I, as well as in Carney triad and Carney-Stratakis syndromes [12,13]. Recently, many centers report epidemiological data demonstrating high occurrence of GISTs with other malignant neoplasms [13–15]. The most frequently associated are gastric and colorectal neoplasms [16,17]. The reported frequency ranges from 2.95 to 33% [17]. Most of these associated GISTs are asymptomatic and found during intraoperative examination of the abdomen [18]. The prognosis of a GIST is based on tumor location, size, and mitotic activity [9–11]. In case of coexistence, accurate staging of both malignancies is very important because the dominant malignancy determines the outcome. The invention of imatinib mesylate opened new perspectives in the treatment of GISTs; especially, its neoadjuvant use helps to downstage inoperable cases and to achieve negative margins resections [19]. To date, researchers have not been able to determine if the association between GIST and colonic tumors is a simple coincidental coexistence or wether the 2 neoplasias are connected by a causal relationship. Attempts have been made to explain the coexistence and simultaneous development of GIST tumor with other gastrointestinal malignancies by studying the c-kit expression in both tumors. It is well known that kit protein can be detected in 80% of benign and 90% of metastatic GIST; it is also believed that mutations of the kit proto-oncogene are the cause of GIST tumors [6,20,21]. There are reports that show 30% c-kit expression in colorectal malignancies [21]. Moreover, it is reported that the c-kit activation in colorectal cancers promotes their invasiveness and metastatic potential [22]. However, there are other reports that contradict the hypothesis of common carcinogenic etiology by showing that the c-kit expression is very rare in colon cancer cell lines [2]. Researchers tried to explain the concurrence of GIST and colorectal cancer by studying the role of metallothionein (MT) in oncogenesis. MTs are coded by 10 genes and play a crucial role in angiogenesis, apoptosis, and cell survival, and are overexposed in lung, ovarian, breast, uterus, pancreas, and skin cancers. However, they are down-regulated in colorectal, hepatic, and gastric cancers [23,24]. MT is over-expressed in all GISTs [25,26]. From all the above findings, genetic pathways seem to be different in these 2 neoplasias. It is obvious that further data are required to support the hypothesis of common carcinogenic etiology.
Conclusions
Accurate staging is essential because the dominant neoplasia usually determines the outcome. Further research is needed to clarify the role of oncogenic mutations and signalling pathways in carcinogenesis of neoplasia of different histiogenic origins.
Abbreviations:
- GIST
gastrointestinal stromal tumor;
- GI
gastrointestinal tract
Footnotes
Conflict of interest
None.
References:
- 1.Wronski M, Ziarkiewicz-Wroblewska B, Gornicka B, et al. Synchronous occurrence of gastrointestinal tumors and other primary gastrointestinal neoplasms. World J Gastroenterol. 2006;12:5360–62. doi: 10.3748/wjg.v12.i33.5360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Melis M, Choi EA, Anders R, et al. Synchronous colorectal adenocarcinoma and gastrointestinal tumor (GIST) Int J Colorectal Dis. 2007;22:109–14. doi: 10.1007/s00384-006-0089-6. [DOI] [PubMed] [Google Scholar]
- 3.Gopal SV, Langcake ME, Johnston E, Salisbury EL. Synchronous association of small bowel stromal tumour with colonic adenocarcinoma. ANZ J Surg. 2008;78:827–28. doi: 10.1111/j.1445-2197.2008.04669.x. [DOI] [PubMed] [Google Scholar]
- 4.Mazur MT, Clark HB. Gastric stromal tumors Reappraisal of histogenesis. Am J Surg Pathol. 1983;7:507–19. doi: 10.1097/00000478-198309000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Rubin BP, Singer S, Tsao C, et al. KIT activation is a ubiquitous feature of gastrointestinal stromal tumors. Cancer Res. 2001;61:8118–21. [PubMed] [Google Scholar]
- 6.Corless CL, McGreevey L, Haley A, et al. KIT mutations are common in incidental gastrointestinal stromal tumors one centimetre or less in size. Am J Pathol. 2002;160:1567–72. doi: 10.1016/S0002-9440(10)61103-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heinrich MC, Corless CL, Duensing A, et al. PDGFRA activating mutations in gastrointestinal stromal tumors. Science. 2003;299:708–10. doi: 10.1126/science.1079666. [DOI] [PubMed] [Google Scholar]
- 8.Agaimy A, Terraciano LM, Dirnhofer S, et al. VG600E BRAF mutations are alternative early molecular events in subset of KIT/PDGFRA wild-type gastrointestinal tumors. J Clin Pathol. 2009;62(7):613–16. doi: 10.1136/jcp.2009.064550. [DOI] [PubMed] [Google Scholar]
- 9.Schneider-Stock R, Boltze C, Lasota J, et al. Loss of p16 protein defines high-risk patients with gastrointestinal stromal tumors: a tissue microarray study. Clin Cancer Res. 2005;11:638–45. [PubMed] [Google Scholar]
- 10.Sabah M, Cummins R, Leader M, et al. Loss of heterozygosity of chromo-some 9p and loss of p16 INK4A expression are associated with malignant gastrointestinal stromal tumors. Mod Pathol. 2004;17:1364–71. doi: 10.1038/modpathol.3800199. [DOI] [PubMed] [Google Scholar]
- 11.Miettinen M, El-Rifai W, HL Sobin L, Lasota J. Evaluation of malignancy and prognosis of gastrointestinal stromal tumors: a review. Hum Pathol. 2002;33:478–83. doi: 10.1053/hupa.2002.124123. [DOI] [PubMed] [Google Scholar]
- 12.Takazawa Y, Sakurai Y, Ikeda T, et al. Gastrointestinal stromal tumours of neurofibromatosis type I (von Recklinghausen’s disease) Am J Surg Pathol. 2005;29:755–63. doi: 10.1097/01.pas.0000163359.32734.f9. [DOI] [PubMed] [Google Scholar]
- 13.Stratakis CA, Carney JA. The triad of paragangliomas, gastric stromal tumours and pulmonary chordomas (carney triad) and the dyad of paragangliomas and gastric stromal sarcomas (carney-stratakis syndrome): molecular genetics and clinical implications. J Inter Med. 2009;266:43–52. doi: 10.1111/j.1365-2796.2009.02110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miettinen M, Sobin LH, Lasota L. Gastrointestinal stromal tumour of the stomach: a clinicopathologic, immunohistochemical, and molecular genetic study of 1765 cases with long term follow-up. Am J Surg Pathol. 2005;29:52–68. doi: 10.1097/01.pas.0000146010.92933.de. [DOI] [PubMed] [Google Scholar]
- 15.Urbanczyk K, Limon J, Korobowicz E, et al. Gastrointestinal stromal tumors. A multicentre experience. Polish Journal of Pathology. 2005;56:51–61. [PubMed] [Google Scholar]
- 16.Arnogiannaki N, Martzoukou I, Kountourakis P, et al. Synchronous presentation of GISTs and other primary neoplasms: a single centre experience. In vivo. 2010;241:109–15. [PubMed] [Google Scholar]
- 17.Agaimy A, Wunsch PH, Sobin LH, et al. Occurrence of other malignancies in patients with gastrointestinal tumors. Sem Diagn Pathol. 2006;23:120–29. doi: 10.1053/j.semdp.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 18.Hassan I, You YN, Dozois EJ, et al. Clinical pathologic and immunohistochemical characteristics of gastrointestinal stromal tumors of the colon and rectum: implications for surgical management and adjuvant therapies. Dis Col Rec. 2006;49:605–15. doi: 10.1007/s10350-006-0503-8. [DOI] [PubMed] [Google Scholar]
- 19.Vassos N, Agaimy A, Hohenberger W, Croner R. Coexistence of gastrointestinal stromal tumors (GIST) and malignant neoplasms of different origin: Prognostic implications. Int J Surg. 2014;12(5):371–77. doi: 10.1016/j.ijsu.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 20.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (versio1.1) Eur J Cancer. 2009;45:228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 21.Sammarco I, Capurso G, Coppola L, et al. Expression of the proto-oncogene C-KIT in normal and tumour tissues from colorectal carcinoma patients. Colorectal Dis. 2004;19:545–53. doi: 10.1007/s00384-004-0601-9. [DOI] [PubMed] [Google Scholar]
- 22.Bellone G, Carbone A, Sibona N, et al. Abberant activation of c-KIT protects colon carcinoma cells against apoptosis and enhances their invasive potential. Cancer Res. 2001;61:2200–6. [PubMed] [Google Scholar]
- 23.Cherian MG, Jayasurya A, Bay BH. Metallothioneins in human tumors and potential roles in carcinogenesis. Mutat Res. 2003;533:203–9. doi: 10.1016/j.mrfmmm.2003.07.013. [DOI] [PubMed] [Google Scholar]
- 24.Padersen M, Larsen A, Stoltenberg M, Penkowa M. The role of metallothionein in oncogenesis and tumour prognosis. Prog Histochem Cytochem. 2009;44:29–64. doi: 10.1016/j.proghi.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 25.Soo ET, Ng CT, Yip GW, et al. Differential expression of metallothionein in gastrointestinal stromal tumors and gastric carcinomas. Anat Rec. 2011;294:267–72. doi: 10.1002/ar.21321. [DOI] [PubMed] [Google Scholar]
- 26.Thirumoorthy N, Shyan Sunder A, Manisenthil Kumar R, et al. A review of metallothionein isoforms and their role in pathophysiology. World J Surg Oncol. 2011;9:54. doi: 10.1186/1477-7819-9-54. [DOI] [PMC free article] [PubMed] [Google Scholar]


