Abstract
Objective
Development of a new framework for the National Institute on Aging (NIA) to assess progress and opportunities toward stimulating and supporting rigorous research to address health disparities.
Design
Portfolio review of NIA’s health disparities research portfolio to evaluate NIA’s progress in addressing priority health disparities areas.
Results
The NIA Health Disparities Research Framework highlights important factors for health disparities research related to aging, provides an organizing structure for tracking progress, stimulates opportunities to better delineate causal pathways and broadens the scope for malleable targets for intervention, aiding in our efforts to address health disparities in the aging population.
Conclusions
The promise of health disparities research depends largely on scientific rigor that builds on past findings and aggressively pursues new approaches. The NIA Health Disparities Framework provides a landscape for stimulating interdisciplinary approaches, evaluating research productivity and identifying opportunities for innovative health disparities research related to aging.
Keywords: Aging, Health Disparities
Introduction
Population-level differences in health status and life expectancy are well-documented.1 While most Americans die from heart disease, various cancers, respiratory disease, stroke and unintentional injuries, there are differences among population-level groups.2 Adolescents and young adults are more likely to die from unintentional injury than middle-aged adults, and older adults are more likely to die from heart disease than adolescents and middle-aged adults. Based on National Vital Statistics for 2013, men have higher age-adjusted mortality than women for heart disease (10%), cancer (20%), unintentional injuries (40%), suicide, and chronic liver disease and lower for stroke (20%) and Alzheimer’s Disease.3 Mortality rates and causes also vary substantially by racial and ethnic classifications among men.4 For example, mortality rates have decreased for all men in the United States, yet there are differences in this decline among racial and ethnic groups.4 Moreover, Table 1 shows differences in specific causes of mortality among racial and ethnic groups of men that may result from both behavioral and biological processes and variations in exposure to social and environmental factors.2, 5 Chronic kidney disease is one example of a condition that disproportionately affects all minority men compared with White men; yet, the two main risk factors of hypertension and diabetes can be controlled.6 Another example is lung cancer that disproportionately affects African Americans and Native Hawaiians, even after adjusting for cigarette smoking intensity.7
Table 1. Leading causes of death by race among US males, 2013.
| Race/Ethnicity | ||||||||||
| White | African American | Latino | Asian/ Pacific Islander | American Indian/ Alaskan Native | ||||||
| Cause of death | % deaths (rank) | AAMa | % deaths (rank) | AAMa | % deaths (rank) | AAMa | % deaths (rank) | AAMa | % deaths (rank) | AAMa |
| Heart disease | 24.8 (1) | 222.6 | 24.0(1) | 177.2 | 20.7 (1) | 66.9 | 23.6 (2) | 81.9 | 19.8 (1) | 82.3 |
| Cancer | 23.7 (2) | 213 | 22.4 (2) | 165.6 | 20.7 (1) | 66.9 | 26.1 (1) | 90.5 | 17.7 (2) | 73.6 |
| Respiratory disease | 5.7 (4) | 51.6 | 3.3 (6) | 24.2 | 2.9 (5) | 9.2 | 3.6 (6) | 12.5 | 4.0 (6) | 16.5 |
| Unintentional injuries | 6.3 (3) | 56.8 | 5.8 (3) | 43.1 | 9.9 (2) | 31.9 | 5.0 (4) | 17.5 | 12.6 (3) | 52.5 |
| Stroke | 4.0 (5) | 35.8 | 4.7 (4) | 35.1 | 4.3 (4) | 14 | 6.1 (3) | 21.3 | 2.7 (7) | 11.3 |
| Alzheimer’s disease | 2.1 (8) | 19.1 | -- | -- | 1.5 (9) | 4.7 | 1.4 (10) | 5 | 0.8 (10) | 3.4 |
| Diabetes | 2.9 (6) | 25.7 | 4.1 (5) | 30.5 | 4.4 (3) | 14.3 | 4.0 (5) | 13.8 | 5.3 (4) | 21.9 |
| Influenza | 2.1 (8) | 18.5 | 1.7 (8) | 12.9 | 2.0 (7) | 6.6 | 3.3 (7) | 11.3 | 2.0 (8) | 8.5 |
| Nephritis | 1.7 (9) | 15.2 | 2.6 (7) | 19 | 1.8 (8) | 5.8 | 1.9 (9) | 6.4 | 1.5 (9) | 6.4 |
| Suicide | 2.6 (7) | 23.4 | 1.2 (9) | 9 | 2.6 (6) | 8.3 | 2.6 (8) | 9.2 | 4.3 (5) | 17.9 |
a. AAM, Age-adjusted mortality, per 100,000 population.
Health disparities research related to aging is the study of biological, behavioral, sociocultural and environmental factors that influence population-level health differences. This research has used a number of approaches to ascertain the underlying determinants of population differences. For example, differential exposure to stress has been identified.8 Other factors, such as socioeconomic status (SES), access to quality health care, and health behaviors have been shown to influence health disparities for American populations, although racial and ethnic disparities in health have not been fully explained.4 Older US racial and ethnic minority populations suffer premature morbidity over the life course, pointing to biological-environmental interactions that hold important implications for understanding mechanisms to explain health disparities. Better understanding of these population differences can lead to developing interventions, critical insights for new therapeutics and public health recommendations that may improve the lives of all elderly populations.5 Thus, research designed to understand, prevent, and ameliorate health disparities should be a public health research priority. Institutions that protect public health by supporting biomedical research should support such research and assess progress toward achieving health equity by reducing and ultimately eliminating health disparities. Thus, one fundamental issue for these institutions is the development of approaches for the analysis and evaluation of health disparities research activities.
This article describes a new framework that the National Institute on Aging (NIA) will use to assess progress in health disparities research. The first section describes the NIA’s impetus for developing priorities in health disparities research. Specifically, we highlight the work of the National Advisory Council on Aging (NACA) and its Task Force on Minority Aging Research to review the NIA’s health disparities research portfolio. Second, we discuss specific levels of analysis that are included in the framework, with hope that consideration of these levels will motivate research that elucidates underlying mechanisms that create and sustain health disparities. Finally, we present our framework to guide NIA’s activities for assessing and evaluating health disparities research.
NIA Health Disparities Portfolio Review
The National Advisory Council on Aging (NACA) meets regularly to advise the director of NIA on its mission. In January 2012, NACA’s Task Force on Minority Aging Research (TFMAR) launched a review of NIA’s health disparities research portfolio and researcher training activities for diverse investigators. The purpose of the review was to evaluate NIA’s progress in addressing priority health disparities areas.
The review was launched by the identification of grants funded from 2002 – 2011 that were coded as health disparities research, clearly studied a health disparities issue, focused on racial/ethnic minorities, low-income populations as primary study participants or were active in the training and recruitment of minority investigators. Nine-hundred eighty-five (985) grants were identified by this process, of which 694 were research focused (ie, were not training or conference grants). The threshold for identifying health disparities research projects was calculated based on the number of specific aims addressing health disparities relative to the total number of aims. For example, if one of four aims for a grant was devoted to this type of research, 25% of the total funding was considered to be devoted to health disparities research. Research grants needed to have a minimum of 10% content dedicated to a health disparities research issue to be included. The vast majority exceeded this threshold based on an independent review of a random subsample. Health disparities research grants represented a modest proportion of all relevant NIA grant awards from 2002-2011 (3.8%). Table 2 displays the variety of funding mechanisms for the 694 research focused grants, including program project/center grants (P01, P30, P50, P60), independent discrete research projects (R01) and exploratory and developmental projects (R21, R03). During the review period, the proportion of NIA grants that were relevant to health disparities research varied by funding mechanism. For example, of all grants focused on health disparities research, almost 58% were R01 funded grants, 13.6% were R03 funded grants and 12.3% were P01, P30, P50, and P60 funded grants.
Table 2. NIA health disparities research portfolio review, 2002-2011.
| Funding Mechanisma | n/N (%) of total health disparities research grants | n/N (%) of total NIA grants funded |
| P01, P30, P50, P60 | 86/694 (12.3) | 86/2215 (3.8) |
| R00, R21, R37, R43, R44, R56 | 71/694 (10.2) | 71/3017 (2.3) |
| R01 | 402/694 (57.9) | 402/10965 (3.7) |
| R03 | 95/694 (13.6) | 95/951 (9.9) |
| RC1, RC2, RC4 | 8/694 (1.1) | 8/153 (5.2) |
| U01 | 28/694 (4.0) | 28/688 (4.1) |
| U13, U24 | 4/694 (.5) | 4/57 (7.0) |
| Total | 694 | 694/18024 (3.8) |
a. P01, research program projects; P30, research program - exploratory projects; P50, research program - specialized center; P60, research program - comprehensive center; R00, research projects - transition award; R01, research projects; R03, research projects - small research grants; R21, research projects - exploratory/developmental grants; R37, research projects - method to extend research in time grant; R43, research projects – small business innovation research grants; R44, research projects – small business innovation research grants (phase 2); R56, research projects - high priority short term research award; RC1, research projects – challenge grants and partnerships program; RC2, research projects - high impact research and infrastructure grants; RC3, research projects - biomedical research, development, and growth for new technologies; U01, cooperative agreement – research project; U13, cooperative agreement – conference grant; U24, cooperative agreement – resource related research projects.
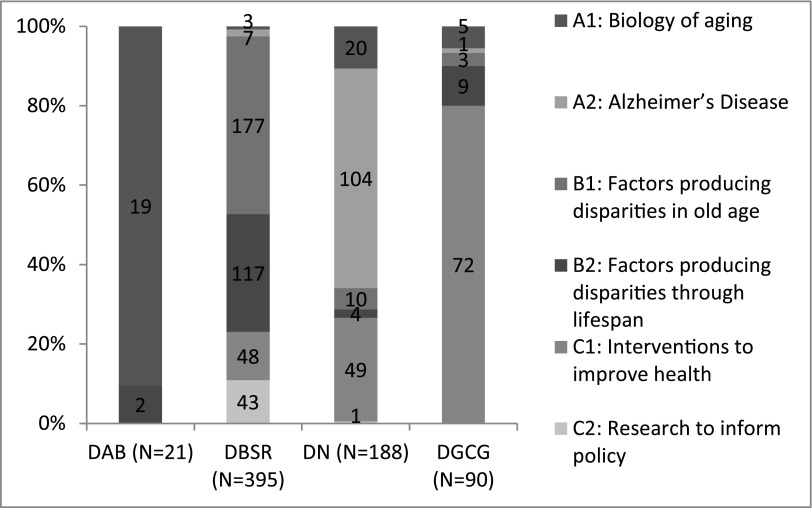
NIA-supported health disparities research grants were also categorized by focus area, including: 1) biology of aging; 2) Alzheimer’s Disease; 3) factors producing disparities in old age; 4) factors producing disparities throughout the lifespan; 5) interventions to improve health; and 6) research to inform policy. The types of grants funded by each NIA division, based on these categories, are shown in Figure 1. The majority were focused on population, social sciences and individual behavioral processes. NIA’s Division of Behavioral and Social Research supported the greatest number of health disparities research projects. These findings motivated NIA’s TFMAR to propose recommendations for stimulating health disparities research across the NIA.
Figure 1. Type of health disparities research by NIA Division.
DAB, Division of Aging Biology; DBSR, Division of Behavioral and Social Research; DN, Division of Neuroscience; DGCG, Division of Geriatrics and Clinical Gerontology.
At the NACA meeting in January, 2013, it was recommended that the NIA regularly examine national data relevant to health disparities, encourage Aging Biology research that informs health disparities, and develop an integrative, conceptual framework to guide health disparities research. This framework should convey health disparities research with an age-related, multidimensional lifecourse approach, and focus on factors operating through specific levels of analyses that are: 1) environmental; 2) sociocultural; 3) behavioral; and 4) biological. The development of this framework was considered to be important for identifying gaps, opportunities and progress in health disparities research at the NIA.
NIA Health Disparities Research Framework
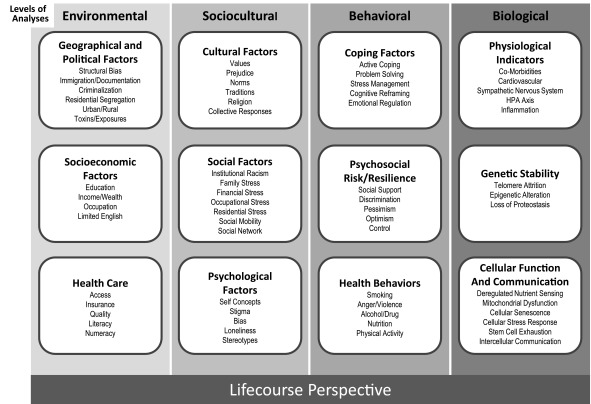
The NIA Health Disparities Research Framework was developed through an iterative process by the authors with feedback from TFMAR members and NIA extramural staff. The framework (Figure 2) represents an effort to organize factors examined in health disparities research related to aging. This framework is important to the NIA for at least three reasons. First, the NIA Health Disparities Framework highlights fundamental factors that determine priority populations for health disparities research, identifying race, ethnicity, socioeconomic status, disability status and gender, sex identity as important to all levels of analysis. Second, the vast range of factors that potentially influence health disparities demand an organizational structure for tracking progress and identifying areas of need. While we note that the various columns of the framework may be organized differently, our purpose is to provide a broad representation of the many factors that should be considered for health disparities research related to aging. Third, the identification of factors at multiple levels of analysis provides the opportunity to better delineate causal pathways, linking environmental, sociocultural, behavioral and biological factors to health and disease. Finally, a consideration of multiple levels of analysis broadens the scope of our search for malleable targets for intervention, aiding in our efforts to ameliorate or reduce health disparities in the aging population.
Figure 2. Fundamental factors: Ethnicity, Gender, Age, Race, Disability Status, Identity.
Levels of Analysis
The factors that determine health of any population - and health differences between populations - can be viewed as occurring at environmental, sociocultural, behavioral, and biological levels.9 The sections below provide descriptions of each of these levels of analysis and include examples of relevant factors for health disparities research related to aging.
Environmental
An environmental level of analysis encompasses a broad spectrum of factors, including exposure to heavy metals and small particulate matter in segregated residential communities that threaten population health.10 Other exposures such as poverty, crime and violence could influence the health of some populations disproportionately and may be related to structural factors that are geographic and political.11,12 In this case, residential segregation that limits opportunities for social mobility constrains some population groups to risky physical environments.13 Immigrant population groups present a unique case, as many immigrants have a healthier profile on entering the United States. Some immigrants experience downward social mobility because they are unable to use the skills they had in their home country, and the acculturation and assimilation process is often not linear. However, populations of immigrants living in ethnic enclaves generally report better health and less stress than their counterparts in other neighborhoods, even with a high proportion with limited English.14
Environmental factors shape opportunities that could influence social class. Socioeconomic status (SES) is the intersection between education, occupation and income15 and can be shaped by important geographical and political factors. In this case, a quality education is needed to obtain gainful employment. Structural bias by race, ethnicity, immigration status and social class may influence local economic support for public schooling.16 Quality indicators such as teacher credentialing, rigorous academic counseling, connections with colleges, universities and school building deterioration are influenced by local tax bases that are linked to residential segregation.16,17 Inadequate educational preparation may weaken chances for college entrance and needed professional training to enter the workforce. High levels of unemployment, underemployment and employment dissatisfaction may result, restricting important resources for protecting health. One very important resource for protecting health is access to quality health care.18 While health care availability and quality are critical for accurate diagnosis and treatment of disease, this resource may be influenced by residential segregation that affects health insurance, quality nursing homes and availability of hospice care.19,20 For example, immigrant populations that migrate to the United States and experience residential segregation may endure barriers to health care because of lack of insurance, not being eligible for government programs and limited access in their area. Furthermore, many immigrants suffer misdiagnosis of disease because of limited English proficiency, numeracy, health literacy and cultural awareness or styles in communication.21
Sociocultural
Group norms, beliefs, values and collective responses are important for considering the impact of environmental risks on population-level differences in health status and life expectancy.22 In the United States, the “American Creed” is an ideology that shapes important collective beliefs about rugged individualism and the importance of high effort performance to obtain important resources, such as power, wealth and optimal health.23 However, a focused lens of American society highlights the existence of many population groups that have endured historical trauma that may influence shared experiences and beliefs about the authenticity of the American Creed.24 Population-level beliefs, attitudes and norms may be shaped by ethnic tradition, migration processes and collective group responses to structural bias that hold important implications for health disparities research.25
Sociocultural factors theorized to influence health disparities include cultural norms, values and social mobility. The inclusion of these factors in the framework emphasizes both the importance of social factors for population health and collective responses to environmental factors that threaten health. First, cultural factors are influential in determining the impact of environmental risks such as inadequate health care and criminalization on population health.25 These cultural factors affect vigilance and confidence that individuals may summon when faced with limited access to health resources. For example, while African Americans have endured chronically low SES,26 structural bias and inadequate access to quality health care,18 this population group has historically created and participated in productive networks of exchange and support. These networks are particularly important for individuals facing harsh circumstances such as unemployment and illness. 27 Investigating sociocultural factors related to this type of group resilience is important for health disparities research related to aging.
Sociocultural norms and values also impact individual-level self-concepts, perceptions, and cognition.28,29 In addition to self-concepts, social identity and perceptions of bias shape individual behavior and may impact both interpersonal relationships and interactions with social institutions to compromise health at the individual level. For example, individuals may identify negative interactions with clinicians to be culturally insensitive, perceive restricted access to quality health care as institutional racism, and view this hardship as discrimination.30 These perceptions may shape important individual-level decisions that further impact health status and life expectancy.
Behavioral
Individual behaviors and psychological processes represent major pathways by which environmental and social exposures impact health. Adverse environmental and sociocultural exposures can tax individual capacity to maintain control and equilibrium, resulting in states of perceived psychosocial stress.31 Individuals differ in the resources that they bring to handle these challenges. Certain psychological capacities, such as problem-solving skills or emotional regulatory strategies represent a spectrum of coping behaviors that can be used to manage demand.32 For example, problem solving behavior motivates individuals to identify and respond to threats. This includes processes like taking direct action, cognitive reframing and seeking social support.31
Individual attitudes, including optimism, pessimism, and sense of control can also serve as additional risk or resilience factors that shape encounters with social and environmental stressors.33 Similarly, quality and types of social support in an individual’s life can serve as a buffer or threat to coping under stressful challenge.34,35 Behaviors, including health behaviors, represent a more direct approach to coping with stressful challenges. Engaging in some behaviors may help regulate emotional responses to social or environmental threats and elevate feelings of control.32 Some of these, such as physical activity, meditation or prayer and social engagement activities such as volunteering may actually be helpful to a certain threshold.36 Others, like tobacco use, alcohol abuse, illegal substance abuse and poor nutritional habits may ignite processes that ultimately diminish health status and when enacted over the lifespan, comprise healthy aging and reduce life expectancy.37 For example, research finds that populations that live in chronically stressful environments, and engage in coping behaviors such as smoking cigarettes, drinking alcohol in excess, and eating poor quality food in excess, also had higher reports of cancer, stroke, hypertension and heart attack.38 In the case of tobacco use, there is unequivocal evidence of harm when used even in small quantities and in fact, second-hand smoke causes disease as an unintended consequence.7 However, for other behaviors with potential adverse consequences, a vexing issue for health disparities research related to aging is to consider these behaviors along with biological processes that impact population health.
Biological
Ultimately, we are interested in the pathways that environmental and sociocultural factors – often transduced through psychological and behavioral processes – become biologically embedded. Health disparities research should prioritize biological processes that help explain mechanisms of observed differences in disease incidence and outcomes that are most relevant for populations that suffer premature mortality and endure harsh environmental conditions with exceptional resilience.39 For example, once stress is perceived, the brain’s hypothalamus sounds an alert to the adrenal glands indicating a need to respond to a potentially harmful threat by the hypothalamus-pituitary-adrenal axis (HPA). As a result, the adrenal glands produce cortisol that replenishes energy stores depleted by the initial adrenaline rush. The HPA ultimately determines whether the stress will be handled by physiologic response or by HPA overload.40 Chronic HPA overload may lead to poor health outcomes such as diabetes mellitus, depression, asthma, and chronic fatigue syndrome.39,40
Chronic stress linked to harsh environmental conditions may influence key aging processes such as cellular stability and telomere stability.41 Telomeres are chromosomal regions that protect the ends of chromosomes from being recognized as DNA damage. The accumulation of genetic, DNA damage throughout life is believed to be central to aging.42 One important question is whether this genetic damage may be accelerated in the face of chronic stress. Rapid attrition of telomere regions has been used as a marker of accelerated aging and mortality risk, providing a gauge for premature development of diseases such as aplastic anemia.43
Telomere shortening leads to a durable dismantling of the cell cycle. This phenomenon, also known as cellular senescence, can be triggered by factors such as DNA damage.44 Cellular senescence can affect the innate arm of the immune response by the active secretion of numerous cytokines and other pro-inflammatory molecules.45 Moreover, cellular senescence disrupts intercellular communication, which is important for the endocrine and nervous systems to function properly and regulate the inflammatory processes for tissues.46 For example, challenges to intercellular communication disrupt the normal secretion of hormones to the bloodstream. This may be especially important for groups of individuals that experience harsh environmental and social arrangements that are perceived as stress, which is handled through various forms of coping behavior that may spark these biological processes to accelerate aging, undermine health status and reduce life expectancy.
The epidemiology of the leading causes of death in the United States by race/ethnic groups reveals striking differences that are not fully explained by the environmental, sociocultural and behavioral factors.42 For example, diabetes mellitus is well-known to be more prevalent among most minority groups, especially Latinos, but there is a clear difference in observed clinical outcomes by race/ethnicity among persons with diabetes that is unexplained. Within a large health care system, Latino, African Americans and Pacific Islander patients with diabetes had 30% lower rates of myocardial infarction, stroke and heart failure at 10 years but a marked increase in the rate of end-stage renal disease relative to Whites.47 In the case of cancer, differences in incidence vary by race/ethnicity by 30% to 50% for breast, prostate and lung cancer. Recently, a protective polymorphism for breast cancer was identified that is present almost exclusively in Latinas with American Indigenous admixture.48 Moreover, other research finds increased survival for racial and ethnic minority populations in the setting of several chronic diseases, suggesting potentially important biologic resilience factors that may protect health.49 However, a recent systematic review found that genome-wide studies contributed scantily to our understanding of racial health disparities in cardiovascular disease.50 Hence, it is important to clarify biological factors along with all of the other previously cited levels of analyses when considering health disparities research related to aging.
Conclusion
The promise of NIA-supported health disparities research depends largely on scientific rigor that builds on past findings and aggressively pursues new approaches.51 This research should encourage contributions from multiple disciplines and promote integrative investigations.52 Moreover, new directions for health disparities research should include multiple levels of analysis, specifically those that are environmental, sociocultural, behavioral and biological.9 NIA-supported projects such as the Healthy Aging in Neighborhoods of Diversity Across the Lifespan Study (HANDLS),53 the Midlife Development in the United States Study (MIDUS),54 and the Health and Retirement Study (HRS)55 provide data that permit these types of analyses. Moreover, NIA-supported investigators are developing interdisciplinary teams to move in this direction.56-59 The NIA Health Disparities Framework provides a landscape for stimulating multidisciplinary approaches, evaluating research productivity and identifying opportunities for health disparities research related to aging that may ultimately achieve health equity.
Acknowledgments
The authors wish to thank the National Advisory Council on Aging Task Force on Minority Aging Research; Samir Sauma, director of the NIA Office of Planning, Analysis, and Evaluation and Timothy Guinn of the NIA Office of Special Populations for invaluable input regarding the portfolio analysis and review of health disparities research at NIA; the National Institute on Aging (NIA) Minority Working Group, particularly Lisbeth Nielsen, Felipe Sierra, John Haaga, Lyndon Joseph, and Robin Barr; staff of the NIA extramural divisions; and Danielle Cloutier and Erin Calhoun for assistance in editing the manuscript.
Carl V. Hill, PhD, MPH is director of the National Institute on Aging’s (NIA) Office of Special Populations. In this role, he directs the NIA Butler-Williams Scholars Program. He is co-chairman of the federal Interagency Committee on Disability Research’s Committee on Health and Health Disparities and chairman of NIH’s Special Populations Research Forum and NIA’s Minority Working Group. Dr. Hill is a graduate of Morehouse College and member of the first graduating class of the Master of Public Health (MPH) Program at Morehouse School of Medicine. Dr. Hill earned his doctoral degree from the University of Michigan’s School of Public Health, under the direction of Harold W. Neighbors, PhD at the Center for Research on Ethnicity, Culture and Health (R25 GM058641). He started his NIH career with the National Center on Minority Health and Health Disparities (now the National Institute on Minority Health and Health Disparities).
Eliseo J. Pérez-Stable, MD is professor of Medicine and chief of the Division of General Internal Medicine, Department of Medicine at the University of California, San Francisco School of Medicine (UCSF). He is the director of the UCSF Medical Effectiveness Research Center for Diverse Populations (MERC) and of the Center for Aging in Diverse Communities, which is funded grant (P30 AG15272) and active through 2017. Dr. Pérez-Stable is a retired member of NIA’s National Advisory Council on Aging, and past chairperson for its Task Force on Minority Aging and Health Disparities Research. In May 2015, Dr. Pérez-Stable was named director of the NIH’s National Institute on Minority Health and Health Disparities (NIMHD) where he will oversee the institute’s activities to improve minority health and reduce health disparities.
Norman B. Anderson, PhD is chief executive officer and executive vice president of the American Psychological Association (APA). Prior to joining APA, Dr. Anderson was the first director of the NIH’s Office of Behavioral and Social Sciences Research (OBSSR). In 2012, Anderson was elected to membership in the National Academy of Medicine (formerly Institute of Medicine) of the National Academy of Science and in September 2013, he was inducted into the National Black College Hall of Fame for his work in science. He is a retired member of NIA’s National Advisory Council on Aging, and served on its Task Force on Minority Aging and Health Disparities Research. A graduate of the North Carolina Central University in Durham, N.C., Dr. Anderson earned both his master’s and doctoral degree in clinical psychology from the University of North Carolina at Greensboro.
Marie A. Bernard, MD is deputy director of the National Institute on Aging (NIA). She co-chairs the Department of Health and Human Services Older Adults Workgroup and the Dementias, Including Alzheimer’s Disease Workgroup for Healthy People 2020. She serves on NIH’s Extramural Activities Working Group, its Diversity Working Group, and co-chairs its Women of Color Committee of the Women in Biomedical Careers Working Group. She was the endowed professor and founding chairman of the Donald W. Reynolds Department of Geriatric Medicine at the University of Oklahoma, College of Medicine. She received her undergraduate education at Bryn Mawr College and her medical degree from University of Pennsylvania. She trained in internal medicine at Temple University Hospital in Philadelphia, PA, where she also served as chief resident.
References
- 1. Williams DR, Yan Yu, Jackson JS, Anderson NB. Racial differences in physical and mental health: socio-economic status, stress and discrimination. J Health Psychol. 1997;2(3):335-351. 10.1177/135910539700200305 [DOI] [PubMed] [Google Scholar]
- 2. Kochanek KD, Murphy SL, Xu J, Arias E. Mortality in the United States, 2013. NCHS Data Brief. 2014;(178):1-8. [PubMed] [Google Scholar]
- 3. Murphy SL, Xu J, Kochanek KD. Deaths: final data for 2010. Natl Vital Stat Rep. 2013;61(4):1-117. [PubMed] [Google Scholar]
- 4. Sandefur G, Campbell ME, Eggerling-Boeck J. Racial and ethnic identification, official classifications, and health disparities. In: Anderson NB, Bulatao RA, Cohen B, eds. Critical Perspectives on Racial and Ethnic Differences in Health in Late Life. Washington, DC: The National Academies Press; 2004:25-52. [PubMed] [Google Scholar]
- 5. Diez Roux AV. Integrating social and biologic factors in health research: a systems view. Ann Epidemiol. 2007;17(7):569-574. 10.1016/j.annepidem.2007.03.001 [DOI] [PubMed] [Google Scholar]
- 6. Flegal KM, Ezzati TM, Harris MI, et al. Prevalence of diabetes in Mexican Americans, Cubans, and Puerto Ricans from the Hispanic health and nutrition examination survey, 1982–1984. Diabetes Care. 1991;14(7):628-638. 10.2337/diacare.14.7.628 [DOI] [PubMed] [Google Scholar]
- 7. Haiman CA, Stram DO, Wilkens LR, et al. Ethnic and racial differences in the smoking-related risk of lung cancer. N Engl J Med. 2006;354(4):333-342. 10.1056/NEJMoa033250 [DOI] [PubMed] [Google Scholar]
- 8. Pearlin LI, Schieman S, Fazio EM, Meersman SC. Stress, health, and the life course: some conceptual perspectives. J Health Soc Behav. 2005;46(2):205-219. 10.1177/002214650504600206 [DOI] [PubMed] [Google Scholar]
- 9. Anderson NB. Levels of analysis in health science. A framework for integrating sociobehavioral and biomedical research. Ann N Y Acad Sci. 1998;840(1):563-576. 10.1111/j.1749-6632.1998.tb09595.x [DOI] [PubMed] [Google Scholar]
- 10. Adeola FO. Endangered community, enduring people: toxic contamination, health, and adaptive responses in a local context. Environ Behav. 2000;32(2):209-249. 10.1177/00139160021972504 [DOI] [Google Scholar]
- 11. Akers TA, Potter RH, Hill CV. Epidemiological criminology: A public health approach to crime and violence. San Francisco, CA: John Wiley & Sons; 2012. [Google Scholar]
- 12. Williams DR, Collins C. Racial residential segregation: a fundamental cause of racial disparities in health. Public Health Rep. 2001;116(5):404-416. 10.1016/S0033-3549(04)50068-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Acevedo-Garcia D, Lochner KA, Osypuk TL, Subramanian SV. Future directions in residential segregation and health research: a multilevel approach. Am J Public Health. 2003;93(2):215-221. 10.2105/AJPH.93.2.215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Haas JS, Phillips KA, Sonneborn D, et al. Variation in access to health care for different racial/ethnic groups by the racial/ethnic composition of an individual’s county of residence. Med Care. 2004;42(7):707-714. 10.1097/01.mlr.0000129906.95881.83 [DOI] [PubMed] [Google Scholar]
- 15. Adler NE, Ostrove JM. Socioeconomic status and health: what we know and what we don’t. Ann N Y Acad Sci. 1999;896(1):3-15. 10.1111/j.1749-6632.1999.tb08101.x [DOI] [PubMed] [Google Scholar]
- 16. Wilson WJ. The truly disadvantaged: The inner city, the underclass, and public policy. Chicago, IL: University of Chicago Press; 2012, 10.7208/chicago/9780226924656.001.0001. [DOI] [Google Scholar]
- 17. Massey DS, Denton NA. The dimensions of residential segregation. Soc Forces. 1988;67(2):281-315. 10.1093/sf/67.2.281 [DOI] [Google Scholar]
- 18. Mayberry RM, Mili F, Ofili E. Racial and ethnic differences in access to medical care. Med Care Res Rev. 2000;57(4)(suppl 1):108-145. 10.1177/107755800773743628 [DOI] [PubMed] [Google Scholar]
- 19. Skinner J, Chandra A, Staiger D, Lee J, McClellan M. Mortality after acute myocardial infarction in hospitals that disproportionately treat black patients. Circulation. 2005;112(17):2634-2641. 10.1161/CIRCULATIONAHA.105.543231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Johnson KS. Racial and ethnic disparities in palliative care. J Palliat Med. 2013;16(11):1329-1334. 10.1089/jpm.2013.9468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vargas Bustamante A, Fang H, Garza J, et al. Variations in healthcare access and utilization among Mexican immigrants: the role of documentation status. J Immigr Minor Health. 2012;14(1):146-155. 10.1007/s10903-010-9406-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pérez-Stable EJ, Nápoles-Springer A, Miramontes JM. The effects of ethnicity and language on medical outcomes of patients with hypertension or diabetes. Med Care. 1997;35(12):1212-1219. 10.1097/00005650-199712000-00005 [DOI] [PubMed] [Google Scholar]
- 23. Geronimus AT, Thompson JP. Racial inequality in health and the policy-induced breakdown of African American communities. In: Saegert S, Freudenberg N, Klitzman, eds. Urban Health and Society: Interdisciplinary Approaches to Research and Practice. San Francisco, CA: Jossey-Bass; 2006.
- 24. Whitbeck LB, Adams GW, Hoyt DR, Chen X. Conceptualizing and measuring historical trauma among American Indian people. Am J Community Psychol. 2004;33(3-4):119-130. 10.1023/B:AJCP.0000027000.77357.31 [DOI] [PubMed] [Google Scholar]
- 25. Airhihenbuwa CO, Liburd L. Eliminating health disparities in the African American population: the interface of culture, gender, and power. Health Educ Behav. 2006;33(4):488-501. 10.1177/1090198106287731 [DOI] [PubMed] [Google Scholar]
- 26. Williams DR, Collins C. US socioeconomic and racial differences in health: patterns and explanations. Annu Rev Sociol. 1995;21(1):349-386. 10.1146/annurev.so.21.080195.002025 [DOI] [Google Scholar]
- 27. Stack CB. All Our Kin: Strategies for Survival in a Black Community. New York, NY: Basic Books; 1975. [Google Scholar]
- 28. Schnittker J, McLeod JD. The social psychology of health disparities. Annu Rev Sociol. 2005;31(1):75-103. 10.1146/annurev.soc.30.012703.110622 [DOI] [Google Scholar]
- 29. Thorpe RJ Jr, Brandon DT, LaVeist TA. Social context as an explanation for race disparities in hypertension: findings from the Exploring Health Disparities in Integrated Communities (EHDIC) Study. Soc Sci Med. 2008;67(10):1604-1611. 10.1016/j.socscimed.2008.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. LaVeist TA, Sellers R, Neighbors HW. Perceived racism and self and system blame attribution: consequences for longevity. Ethn Dis. 2001;11(4):711-721. [PubMed] [Google Scholar]
- 31. Folkman S, Schaefer C, Lazarus RS. Cognitive processes as mediators of stress and coping. Human Stress and Cognition; 1979:265-298. [Google Scholar]
- 32. Cohen F. Measurement of coping. In: Kasl S, Cooper CL, eds. Research methods in stress and health psychology. New York, NY: John Wiley & Sons; 1987. [Google Scholar]
- 33. Myers HF. Ethnicity- and socio-economic status-related stresses in context: an integrative review and conceptual model. J Behav Med. 2009;32(1):9-19. 10.1007/s10865-008-9181-4 [DOI] [PubMed] [Google Scholar]
- 34. Cohen S. Social relationships and health. Am Psychol. 2004;59(8):676-684. 10.1037/0003-066X.59.8.676 [DOI] [PubMed] [Google Scholar]
- 35. Antonucci TC, Jackson JS. Social support, interpersonal efficacy, and health: A life course perspective. In: Carstensen L, Edelstein BA, eds. Handbook of clinical gerontology. Elmsford, NY: Pergamon Press; 1987. [Google Scholar]
- 36. Salmon P. Effects of physical exercise on anxiety, depression, and sensitivity to stress: a unifying theory. Clin Psychol Rev. 2001;21(1):33-61. 10.1016/S0272-7358(99)00032-X [DOI] [PubMed] [Google Scholar]
- 37. Jackson JS, Knight KM, Rafferty JA. Race and unhealthy behaviors: chronic stress, the HPA axis, and physical and mental health disparities over the life course. Am J Public Health. 2010;100(5):933-939. 10.2105/AJPH.2008.143446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Winkleby MA, Cubbin C. Changing patterns in health behaviors and risk factors related to chronic diseases, 1990-2000. Am J Health Promot. 2004;19(1):19-27. 10.4278/0890-1171-19.1.19 [DOI] [PubMed] [Google Scholar]
- 39. Anderson NB, McNeilly M, Myers H. Toward understanding race difference in autonomic reactivity. In: Turner JR, Sherwood A, Light KC, eds. Individual Differences in Cardiovascular Response to Stress. New York, NY: Springer; 1992:125-145, 10.1007/978-1-4899-0697-7_7. [DOI] [Google Scholar]
- 40. McEwen BS, Lasley EN. The end of stress as we know it. Washington, DC: Joseph Henry Press; 2002. [Google Scholar]
- 41. Kyrou I, Tsigos C. Stress hormones: physiological stress and regulation of metabolism. Curr Opin Pharmacol. 2009;9(6):787-793. 10.1016/j.coph.2009.08.007 [DOI] [PubMed] [Google Scholar]
- 42. López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153(6):1194-1217. 10.1016/j.cell.2013.05.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Blackburn EH, Greider CW, Szostak JW. Telomeres and telomerase: the path from maize, Tetrahymena and yeast to human cancer and aging. Nat Med. 2006;12(10):1133-1138. 10.1038/nm1006-1133 [DOI] [PubMed] [Google Scholar]
- 44.>Hayflick L, Moorhead PS. The serial cultivation of human diploid cell strains. Exp Cell Res. 1961;25(3):585-621. 10.1016/0014-4827(61)90192-6 [DOI] [PubMed] [Google Scholar]
- 45. Bodnar AG, Ouellette M, Frolkis M, et al. Extension of life-span by introduction of telomerase into normal human cells. Science. 1998;279(5349):349-352. 10.1126/science.279.5349.349 [DOI] [PubMed] [Google Scholar]
- 46. Salminen A, Ojala J, Kaarniranta K, Kauppinen A. Mitochondrial dysfunction and oxidative stress activate inflammasomes: impact on the aging process and age-related diseases. Cell Mol Life Sci. 2012;69(18):2999-3013. 10.1007/s00018-012-0962-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Karter AJ, Ferrara A, Liu JY, Moffet HH, Ackerson LM, Selby JV. Ethnic disparities in diabetic complications in an insured population. JAMA. 2002;287(19):2519-2527. 10.1001/jama.287.19.2519 [DOI] [PubMed] [Google Scholar]
- 48. Fejerman L, Ahmadiyeh N, Hu D, et al. ; COLUMBUS Consortium . Genome-wide association study of breast cancer in Latinas identifies novel protective variants on 6q25. Nat Commun. 2014;5:5260. 10.1038/ncomms6260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Burrows NR, Cho P, McKeever Bullard K, Narva AS, Eggers PW. Survival on dialysis among American Indians and Alaska Natives with diabetes in the United States, 1995-2010. Am J Public Health. 2014;104(S3)(suppl 3):S490-S495. 10.2105/AJPH.2014.301942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kaufman JS, Dolman L, Rushani D, Cooper RS. The contribution of genomic research to explaining racial disparities in cardiovascular disease: a systematic review. Am J Epidemiol. 2015;181(7):464-472. 10.1093/aje/kwu319 [DOI] [PubMed] [Google Scholar]
- 51. Suzman R, Beard JR, Boerma T, Chatterji S. Health in an ageing world--what do we know? Lancet. 2015;385(9967):484-486. 10.1016/S0140-6736(14)61597-X [DOI] [PubMed] [Google Scholar]
- 52. Hall KL, Feng AX, Moser RP, Stokols D, Taylor BK. Moving the science of team science forward: collaboration and creativity. Am J Prev Med. 2008;35(2)(suppl):S243-S249. 10.1016/j.amepre.2008.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Evans MK, Lepkowski JM, Powe NR, LaVeist T, Kuczmarski MF, Zonderman AB. Healthy aging in neighborhoods of diversity across the life span (HANDLS): overcoming barriers to implementing a longitudinal, epidemiologic, urban study of health, race, and socioeconomic status. Ethn Dis. 2010;20(3):267-275. [PMC free article] [PubMed] [Google Scholar]
- 54. Dienberg Love G, Seeman TE, Weinstein M, Ryff CD. Bioindicators in the MIDUS national study: protocol, measures, sample, and comparative context. J Aging Health. 2010;22(8):1059-1080. 10.1177/0898264310374355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Juster FT, Suzman R. An overview of the Health and Retirement Study. J Hum Resour. 1995;30:S7-S56. 10.2307/146277 [DOI] [Google Scholar]
- 56. Chae DH, Nuru-Jeter AM, Adler NE, et al. Discrimination, racial bias, and telomere length in African-American men. Am J Prev Med. 2014;46(2):103-111. 10.1016/j.amepre.2013.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Needham BL, Adler N, Gregorich S, et al. Socioeconomic status, health behavior, and leukocyte telomere length in the National Health and Nutrition Examination Survey, 1999-2002. Soc Sci Med. 2013;85:1-8. 10.1016/j.socscimed.2013.02.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Missinne S, Neels K, Bracke P. Reconsidering inequalities in preventive health care: an application of cultural health capital theory and the life-course perspective to the take-up of mammography screening. Sociol Health Illn. 2014;36(8):1259-1275. 10.1111/1467-9566.12169 [DOI] [PubMed] [Google Scholar]
- 59. Taylor JY, Wu CY, Darling D, Sun YV, Kardia SL, Jackson JS. Gene-environment effects of SLC4A5 and skin color on blood pressure among African American women. Ethn Dis. 2012;22(2):155-161. [PMC free article] [PubMed] [Google Scholar]