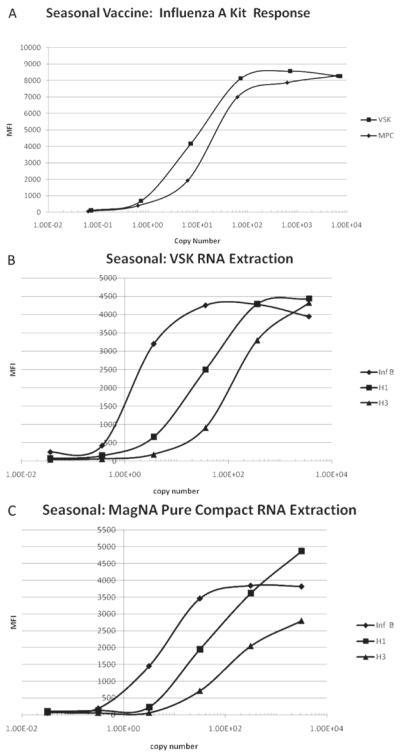
Figure 3.
(A) Influenza A response of the RVP kit versus influenza A dilution calculated copy number for the 2005–2006 seasonal influenza vaccine. The RNA from the samples was extracted with the VSK and MPC. The response is given as MFI as measured by the BioPlex reader. The copy number was calculated by multiplying the concentration of the diluted vaccine by the volume of sample used in the RT-PCR step of the assay, which was 5 μL. The concentration of the diluted vaccine was determined by multiplying the undiluted vaccine concentration by the dilution factor. (B). Influenza B, H1 influenza A subtype, and H3 influenza A subtype response of the RVP kit versus dilution calculated copy number for the 2005–2006 seasonal influenza vaccine for samples extracted with VSK. The response is given as MFI as measured by the BioPlex reader. The copy number was calculated by multiplying the concentration of the diluted vaccine by the volume of sample used in the RT-PCR step of the assay, which was 5 μL. The concentration of the diluted vaccine was determined by multiplying the undiluted vaccine concentration by the dilution factor. (C). Influenza B, H1 influenza A subtype, and H3 influenza A subtype response of Luminex xTAG RVP kit versus dilution calculated copy number for the 2005–2006 seasonal influenza vaccine for samples extracted with MPC. The response is given as MFI as measured by the BioPlex reader. The copy number was calculated by multiplying the concentration of the diluted vaccine by the volume of sample used in the RT-PCR step of the assay, which was 5 μL. The concentration of the diluted vaccine was determined by multiplying the undiluted vaccine concentration by the dilution factor.