Table 2.
Chroman formationa
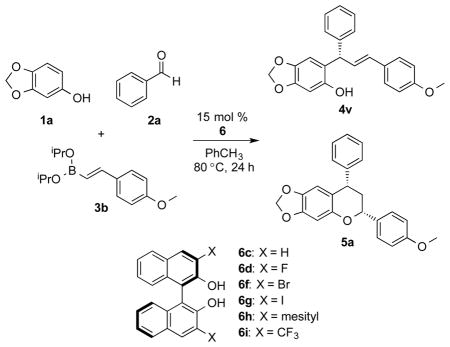
| |||||
|---|---|---|---|---|---|
| entry | catalyst 6 | product 4v yield [%] | product 4v er | product 5a yield [%] | product 5a er |
| 1 | 6c | 22 | 70:30 | 28 | 60:40 |
| 2 | 6d | 46 | 66:33 | 33 | 60:40 |
| 3 | 6f | 22 | 80:20 | 55 | 79:21 |
| 4 | 6g | 31 | 82:18 | 59 | 87:13 |
| 5b | 6h | 37 | 21:79 | 16 | 49:51 |
| 6 | 6i | 39 | 89:11 | 50 | 81:19 |
| 7c | 6g | 5 | - | 69 | 84:16 |
| 8d | 6g | - | - | 72 | 85:15 |
| 9d,e | 6g | - | - | 40 | 99:1 |
Reactions were run at 80 °C with 0.4 mmol phenol, 0.8 mmol aldehyde, and 0.8 mmol boronate for 24 h in toluene (0.3 M) unless otherwise indicated. Diastereomeric ratios were 2:1 as determined by 1H NMR unless otherwise indicated. Enantiomeric ratios are reported for the major diastereomer.
(S)-enantiomer of catalyst used. Mesityl = 2,4,6-trimethylbenzene.
Reaction was run in sealed tube at 120 °C for 24 h.
Reaction was run in a sealed tube at 80 °C for 24 h and then 150 °C for 1 h.
Product was recrystallized from hot hexanes. The diastereomeric ratio was >20:1 as determined by 1H NMR.
