Abstract
Crohn’s disease and ulcerative colitis are the primary inflammatory bowel diseases (IBDs) affecting the gastrointestinal tract. The current therapy aims at decreasing inflammation and reducing symptoms. This typically requires immune suppression by steroids, thiopurines, methotrexate, or tumor necrosis factor inhibitors. Patients may be unreceptive to medical therapy, and some may discontinue the treatment due to adverse effects. Noninvasive, transcutaneous vagus nerve stimulation (VNS) is currently used as a treatment for depression and epilepsy, and it is being investigated for the treatment of conditions such as multiple sclerosis, migraines, and Alzheimer’s disease. Recent studies have demonstrated the importance of splenic and vagus nerve functions in the inflammatory process through the production of certain cytokines. We hypothesize that using transcutaneous VNS via the auricular afferent branch could achieve a selective anti-inflammatory effect on the intestinal wall. This review examines the possibility of using vagal stimulators as a therapy for IBD. This could open the door to novel treatments for numerous vagally mediated diseases characterized by poor responses to current therapies.
Keywords: Crohn’s disease, ulcerative colitis, inflammatory bowel disease, inflammation, vagus nerve stimulation, noninvasive, transcutaneous electrical stimulation
Introduction
Current treatments for inflammatory bowel disease (IBD), including Crohn’s disease and ulcerative colitis, are primarily biological, but these therapies can have serious side effects and limited action, or they may simply be cost prohibitive.1 Recent evidence suggests that reducing inflammation while stimulating mucosal healing may provide the best outcomes.2 Studies have shown that consistent therapy with anti-tumor necrosis factor (TNF) agents will increase the likelihood of mucosal healing and clinical remission, reduce the need for surgery and hospitalization, and avoid the chronic need for corticosteroids.3
It has been demonstrated that the parasympathetic nervous system possesses an anti-inflammatory pathway that inhibits the release of proinflammatory cytokines, including TNF.4 It has been further demonstrated that direct electrical stimulation of the vagus nerve can stimulate this anti-inflammatory mechanism in the gastrointestinal tract through interactions with the enteric nervous system.5 As transcutaneous stimulation of the vagus nerve is already being used in the treatment of epilepsy, it is reasonable to consider the possibility that noninvasive, transcutaneous vagus nerve stimulation (VNS) of the auricular branch of the vagus nerve (ABVN) could potentially reduce the vagal-mediated symptoms of Crohn’s disease, ulcerative colitis, and other chronic IBDs and establish a safer and more affordable treatment modality.
Current Biological Treatment of IBD
Corticosteroids
Corticosteroids suppress activation of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB). This is a primary transcription factor for both the innate and adaptive immune systems in regulating an inflammatory response.6,7 Diabetes, osteoporosis, steroid-induced psychosis, steroid dependence, and opportunistic infections are among the most common side effects.6,7 Corticosteroid use is limited due to its relative inability to induce remission or induce mucosal healing with chronic use.6,8
Thiopurines
Thiopurines are immunosuppressive drugs that act by disabling T lymphocytes involved in the process of inflammation. Azathioprine and other thiopurines act through a complex set of events in which their metabolites are incorporated into the DNA cell lines of rapidly dividing inflammatory cells.9 Azathioprine targets the activation of Rac1, a small guanosine triphosphate phosphohydrolase (GTPase) essential for the activation of T cells in the gastrointestinal tract. This leads to a decrease in NF-κB activation and induces T cell apoptosis, thereby decreasing proinflammatory secretion of cytokines.9 Possible side effects of thiopurine treatment include liver toxicity and the development of lymphoma, which appear to be related to the incorporation of DNA metabolites.9
Methotrexate
Methotrexate is used as an alternative for patients who have been unresponsive to thiopurines. In 2011, Kozarek et al showed that in patients failing to respond to treatment with azathioprine, methotrexate was effective to help maintain the clinical benefits in one year in 63% of patients.10 In this group of patients, 26% discontinued therapy due to side effects. The study demonstrates that therapy with methotrexate is well tolerated and a reasonable option for patients who are unable to receive thiopurines. Methotrexate has a lower rate of mucosal healing compared with that of biologics and azathioprine.6,10
Some common adverse side effects of methotrexate include leucopenia, nausea, anorexia, stomatitis, and diarrhea. More serious effects include bone marrow suppression, hepatic fibrosis, or, rarely, hypersensitivity pneumonia.11,12 Methotrexate is contraindicated in pregnancy due to its known teratogenic effect on the fetus.13
Anti-TNF-α inhibitor therapy
Cytokines are involved in stimulating the acute phase reaction that stimulates the inflammatory cascade. TNF-α is one of the main cytokines involved in the inflammatory cascade. Antigen-presenting cells help initiate an inflammatory response to bacterial products and lipopolysaccharides (LPSs) through the activation of macrophages, T cells, and natural killer cells and the production of TNF-α.6,14 Patients with IBD have an increased number of TNF-α-secreting T cells located in the lamina propria of the intestines.6,14
TNF-α inhibitors have improved the treatment of inflammatory conditions such as the seronegative spondyloarthropathies, rheumatoid arthritis, and IBD.15 In clinical trials and postmarketing surveillance, a number of multiple adverse effects have been identified, including infusion reactions, neutropenia, demyelinating disease, heart failure, infections, injection site reactions, cutaneous reactions, induction of autoimmunity, and malignancy.15 In addition to these adverse effects, a significant number of patients with IBD, being treated with biological agents, lose response over time or do not respond at all.16
Monoclonal antibodies targeting leukocyte migration
Adhesion molecules help leukocytes migrate out of the blood into the site of inflammation.17 Natalizumab, a monoclonal antibody targeting the α4 integrin, was the first drug developed in this category to treat IBD.6,18 A major setback of natalizumab therapy was its interaction with α4β1 vascular cell adhesion molecule-1, which is required by the main effector T cells used to help contain the John Cunningham virus (a human polyomavirus) and prevent brain infections. Newer therapies were developed to help specifically target α4β7 in the vasculature of the gut to avoid the risk of progressive multifocal leukoencephalopathy.6,18
Vagus Nerve
There are two main branches of the autonomic nervous system based on function and anatomy.19 These two divisions are the sympathetic and parasympathetic systems. They help control and regulate organ function through a set of connected neurons, located in either the central nervous system or the peripheral ganglia.19 The parasympathetic neurons arise in the brainstem medulla and the lower sacral portion of the spinal cord, while the peripheral ganglia are located in close proximity to the organ they innervate or within the organ itself.19 The sympathetic neurons begin in the thoracic and lumbar regions of the spinal cord, and the ganglia are located along a chain in close proximity to the spinal cord.19 These two systems counteract one another and help facilitate equilibrium through physiological and environmental responses.19 One such equilibrium regulated in part by the autonomic nervous system is hemostasis, which can deviate from equilibrium during infection due to the release of certain proinflammatory cytokines, such as TNF and interleukin-1β (IL-1β), that mediate fever, shock, and response to tissue injury.19,20
Anti-inflammatory Cholinergic Pathway
In 2000, Borovikova et al4 described how the parasympathetic nervous system has an anti-inflammatory response pathway in response to endotoxin. In human-cultured macrophages, they were able to use acetylcholine and nicotine to diminish the release of proinflammatory cytokines, such as TNF, IL-1β, IL-6, and IL-18. They also demonstrated in rat models that the direct stimulation of the peripheral vagus nerve in vivo during endotoxemia caused an inhibition of TNF synthesis in the liver. This inhibition of TNF synthesis decreased peak serum levels and prevented the development of shock.4 A significantly decreased amount of TNF was present in the serum levels of rats receiving efferent VNS. Vagotomized rats given the same lethal dose of LPS without an electrical stimulation had a demonstrable increased serum concentration of TNF. These data directly connect efferent vagus nerve signaling in the regulatory process of TNF production.4
Two years after the discoveries by Borovikova’s team, Wang et al21 identified the α7 nicotinic acetylcholine receptor (α7nAChR) of splenic macrophages as the mechanism by which parasympathetic stimulation reduces proinflammatory cytokine release. They found that electrical stimulation of the vagus nerve produced a decrease in the production of TNF by macrophages in wild-type mice, while the same stimulation in α7 knockout mice did not decrease TNF production. In 2005, de Jonge et al22 used a murine model of postoperative ileus to examine the anti-inflammatory effects of VNS. Postoperative ileus is associated with general hypomotility and delayed emptying of the gastrointestinal tract.23 It typically results from inflammation caused by the activation of macrophages via physical manipulation of the bowels during intraperitoneal surgery. de Jonge et al22 found that perioperative stimulation of the vagus nerve significantly attenuated the severity of postoperative ileus by STAT3 in the macrophages responsible for the inflammation.
Matteoli et al5 demonstrated the mechanisms by which the vagus nerve directly interacts with the intestines to inhibit inflammation without the involvement of the spleen.5 Inside the intestinal wall, the resident macrophages lie in close vicinity to cholinergic nerve fibers innervated by the vagus nerve via enteric neurons. In order for the vagus nerve to have a protective effect on the intestinal lining, α7nAChR on the macrophages must be expressed. Matteoli et al induced intestinal inflammation in mice devoid of splenic innervation. While stimulating the vagus nerve, they were able to achieve a reduction in intestinal inflammation. Based on their research, it can be concluded that the vagal anti-inflammatory response is in direct contact with the intestines and independent of the spleen.5 The neural response explained earlier seems to be more involved in mediating local inflammatory processes with limited systemic influence, suggesting the idea of neuromodulation.5 The role of the enteric nervous system—based on indirect evidence—shows that myenteric plexus abnormalities have been reported in patients with IBD.5 It has been reported that myenteric plexitis is linked to an increased risk for the development and recurrence of Crohn’s disease in surgically treated patients, suggesting a possible association of the enteric nervous system in the pathology of this illness.24–27
Therapeutic VNS
VNS has been approved for the treatment of refractory epilepsy and depression by the US Food and Drug Administration.28,29 The first VNS device required a surgical implantation of the stimulator and electrodes. The most serious adverse effects associated with implantation were infection, lesions of the vagus nerve, dyspnea, and the need for battery replacement. These symptoms could be related to the possible mechanical failure of an electronic-equipment-based system.30 According to the American Association of Neurological Surgeons,31 side effects of VNS implantation devices that are most commonly used for epilepsy include hoarseness, coughing, throat tickling, and shortness of breath. They assert that these side effects are temporary and eventually resolve with time.
Currently, there are noninvasive VNS systems that improve the safety and tolerability of these devices, allowing the investigation of their usefulness in a wider range of disease processes. A study by Kreuzer et al32 was conducted in 2012 on the impact of transcutaneous stimulation of ABVN in the heart. The study found no indication of arrhythmias in patients without a history of cardiac pathology. Similarly, in 2015, Frangosa et al33 used functional magnetic resonance imaging to prove that transcutaneous stimulation of the ABVN produced significant activation of the areas of the brain known to have central projections from the vagus nerve, including the nucleus tractus solitarii (NTS).
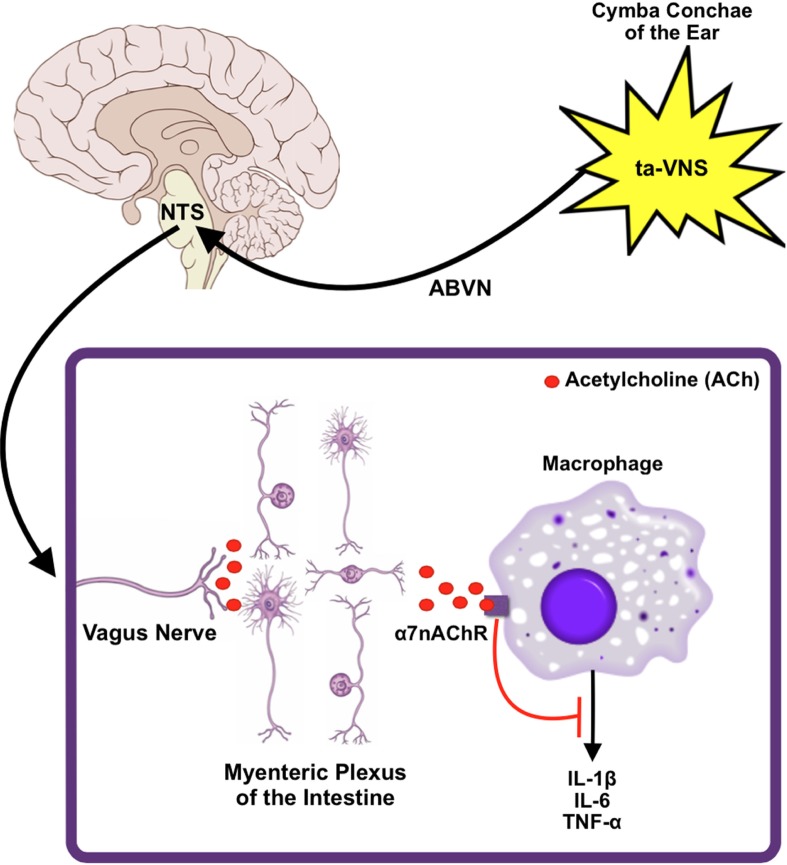
Zhao et al34 demonstrated the effectiveness of transauricular stimulation of the ABVN to illicit an anti-inflammatory effect that facilitated immune suppression in an endotoxemic rat model. The study showed that transauricular stimulation of the ABVN decreased the serum levels of inflammatory cytokines such as TNF-α, IL-1β, and IL-6, as well as the proinflammatory transcription factor such as NF-κB. These anti-inflammatory effects could not be seen in rat models that underwent vagotomy or administration of an α7nAChR antagonist. The proposed model for the anti-inflammatory effects seen in the transauricular stimulation of the ABVN can be summarized thus as follows (Fig. 1): the stimulation of sensory afferent fibers of the ABVN ascends to the NTS, where they synapse and cause the activation of efferent vagus nerve fibers to subdue peripheral cytokine release through the activation of α7nAChR on peripheral macrophages.34
Figure 1.
A proposed model of VNS anti-inflammatory mechanism. Transauricular VNS at the cymba conchae of the ear excites the ABVN. This signal propagates centrally to the NTS and then on to the myenteric plexus of the intestine via the efferent limb of the vagus nerve. Subsequent cholinergic stimulation of the α7nAChR on resident macrophages results in the inhibition of key proinflammatory cytokines.
Conclusion
The current therapy for IBD aims at decreasing systemic inflammation. This approach requires the use of systemic immune suppression such as steroids, thiopurines, methotrexate, and TNF inhibitors. TNF modulators have proven to be very effective in 30–60% of Crohn’s disease patients, but costs are extreme and side effects and adverse events are not minimal. Animal studies have demonstrated that VNS can block proinflammatory TNF secretion by the liver and the gut. Currently, noninvasive transcutaneous VNS is being used as an adjuvant therapy for epilepsy. There exists the potential for use of noninvasive, transcutaneous vagal stimulation to decrease the gastrointestinal inflammation in humans with IBD using the technology that is already available. This new approach could provide clinicians the ability to offer a safe, non-pharmacological adjuvant therapy or patients who have failed the currently approved medical therapy a unique treatment option.
Footnotes
ACADEMIC EDITOR: Melpakkam Srinivas, Editor in Chief
PEER REVIEW: Three peer reviewers contributed to the peer review report. Reviewers’ reports totaled 840 words, excluding any confidential comments to the academic editor.
FUNDING: This study was funded by the Office of the Vice Chancellor of Research, the University of Mississippi Medical Center. The authors confirm that the funder had no influence over the study design, content of the article, or selection of this journal.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Conceived and designed the experiments: TLA, NG, IE, CL, and CRG. Wrote the first draft of the manuscript: RM. Contributed to the writing of the manuscript: RM and IT. Jointly developed the structure and arguments for the article: RM and IT. Made critical revisions and approved the final version: CRG. All authors reviewed and approved the final manuscript.
REFERENCES
- 1.Antunes O, Filippi J, Hebuterne X, Peyrin-Biroulet L. Treatment algorithms in Crohn’s: up, down or something else? Best Pract Res Clin Gastroenterol. 2014;28:473–483. doi: 10.1016/j.bpg.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 2.Peyrin-Biroulet L, Reinisch W, Colombel JF, et al. Clinical disease activity, C-reactive protein normalisation and mucosal healing in Crohn’s disease in the SONIC trial. Gut. 2014;63:88–95. doi: 10.1136/gutjnl-2013-304984. [DOI] [PubMed] [Google Scholar]
- 3.Mandel MD, Miheller P, Mullner K, Golovics PA, Lakatos PL. Have biologics changed the natural history of Crohn’s disease? Dig Dis. 2014;32:351–359. doi: 10.1159/000358135. [DOI] [PubMed] [Google Scholar]
- 4.Borovikova LV, Ivanova S, Zhang M, et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405:458–462. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- 5.Matteoli G, Gomez-Pinilla PJ, Nemethova A, et al. A distinct vagal anti-inflammatory pathway modulates intestinal muscularis resident macrophages independent of the spleen. Gut. 2014;63:938–948. doi: 10.1136/gutjnl-2013-304676. [DOI] [PubMed] [Google Scholar]
- 6.Krishnareddy S, Swaminath A. When combination therapy isn’t working: emerging therapies for the management of inflammatory bowel disease. World J Gastroenterol. 2014;20(5):1139–1146. doi: 10.3748/wjg.v20.i5.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Warren BF, Watkins PE. Effect of steroids in an IBD model. Aliment Pharmacol Ther. 1993;7:585–586. doi: 10.1111/j.1365-2036.1993.tb00078.x. [DOI] [PubMed] [Google Scholar]
- 8.Lukáš K, Dastych M, Novotný A, Prokopová L, Zbořil V. Long-term therapy of idiopathic inflammatory bowel disease. Cas Lek Cesk. 2012;151:231–242. [PubMed] [Google Scholar]
- 9.Neurath M. Thiopurines in IBD: what is their mechanism of action? Gastroenterol Hepatol (N Y) 2010;6:435–436. [PMC free article] [PubMed] [Google Scholar]
- 10.Kozarek RA, Patterson DJ, Gelfand MD, Botoman VA, Ball TJ, Wilske KR. Methotrexate induces clinical and histologic remission in patients with refractory inflammatory bowel disease. Ann Intern Med. 1989;110:353–356. doi: 10.7326/0003-4819-110-5-353. [DOI] [PubMed] [Google Scholar]
- 11.Stein RB, Hanauer SB. Comparative tolerability of treatments for inflammatory bowel disease. Drug Saf. 2000;23:429–448. doi: 10.2165/00002018-200023050-00006. [DOI] [PubMed] [Google Scholar]
- 12.Herfarth HH, Osterman MT. Efficacy of methotrexate in ulcerative colitis: failure or promise. Inflamm Bowel Dis. 2010;16(8):1421–1430. doi: 10.1002/ibd.21246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lewden B, Vial T, Elefant E, et al. French Network of Regional Pharmacovigilance Centers Low dose methotrexate in the first trimester of pregnancy: results of a French collaborative study. J Rheumatol. 2004;31:2360–2365. [PubMed] [Google Scholar]
- 14.D’Haens G Daperno M. Advances in biologic therapy for ulcerative colitis and Crohn’s disease. Curr Gastroenterol Rep. 2006;8:506–512. doi: 10.1007/s11894-006-0041-5. [DOI] [PubMed] [Google Scholar]
- 15.Stone JH, Furst DE, Romain PL. Tumor necrosis factor-alpha inhibitors: an overview of adverse effects. In: Post TW, editor. UpToDate. Waltham, MA: UpToDate; 2015. [Accessed April 3 2015]. Available at: www.uptodate.com. [Google Scholar]
- 16.Yanai H, Hanauer SB. Assessing response and loss of response to biological therapies in IBD. Am J Gastroenterol. 2011;106:685–698. doi: 10.1038/ajg.2011.103. [DOI] [PubMed] [Google Scholar]
- 17.Perrier C, Rutgeerts P. New drug therapies on the horizon for IBD. Dig Dis. 2012;30(suppl 1):100–105. doi: 10.1159/000341133. [DOI] [PubMed] [Google Scholar]
- 18.Allen PB. Anti-adhesion molecules: is gut specificity the key for a good safety profile? Curr Drug Deliv. 2012;9:333–337. doi: 10.2174/156720112801323143. [DOI] [PubMed] [Google Scholar]
- 19.Rosas-Ballina M, Ochani M, Parrish WR, et al. Splenic nerve is required for cholinergic anti-inflammatory pathway control of TNF in endotoxemia. Proc Natl Acad Sci U S A. 2008;105(31):11008–11013. doi: 10.1073/pnas.0803237105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nathan C. Points of control in inflammation. Nature. 2002;420:846–852. doi: 10.1038/nature01320. [DOI] [PubMed] [Google Scholar]
- 21.Wang H, Yu M, Ochani M, et al. Nicotinic acetylcholine receptor α7 subunit is an essential regulator of inflammation. Nature. 2003;421:384–388. doi: 10.1038/nature01339. [DOI] [PubMed] [Google Scholar]
- 22.de Jonge WJ, van der Zanden EP, The FO, et al. Stimulation of the vagus nerve attenuates macrophage activation by activating the Jak2-STAT3 signaling pathway. Nat Immunol. 2005;6:844–851. doi: 10.1038/ni1229. [DOI] [PubMed] [Google Scholar]
- 23.Livingston EH, Passaro EP., Jr Postoperative ileus. Dig Dis Sci. 1990;35:121–132. doi: 10.1007/BF01537233. [DOI] [PubMed] [Google Scholar]
- 24.Sonsino E, Mouy R, Foucaud P, et al. Intestinal pseudoobstruction related to cytomegalovirus infection of myenteric plexus. N Engl J Med. 1984;311:196–197. doi: 10.1056/NEJM198407193110319. [DOI] [PubMed] [Google Scholar]
- 25.Bogers J, Moreels T, De Man J, et al. Schistosoma mansoni infection causing diffuse enteric inflammation and damage of the enteric nervous system in the mouse small intestine. Neurogastroenterol Motil. 2000;12:431–440. doi: 10.1046/j.1365-2982.2000.00219.x. [DOI] [PubMed] [Google Scholar]
- 26.DeSchryver-Kecskemeti K, Clouse RE. Perineural and intraneural inflammatory infiltrates in the intestines of patients with systemic connectivetissue disease. Arch Pathol Lab Med. 1989;113:394–398. [PubMed] [Google Scholar]
- 27.Ferrante M, de Hertogh G, Hlavaty T, et al. The value of myenteric plexitis to predict early postoperative Crohn’s disease recurrence. Gastroenterology. 2006;130:1595–1606. doi: 10.1053/j.gastro.2006.02.025. [DOI] [PubMed] [Google Scholar]
- 28.Shiozawa P, da Silva ME, de Carvalho TC, Cordeiro Q, Brunoni AR, Fregni F. Vagus nerve stimulation in the treatment of epilepsy. Arq Neuropsiquiatr. 2014;72:542–547. doi: 10.1590/0004-282x20140061. [DOI] [PubMed] [Google Scholar]
- 29.Grimm S, Bajbouj M. Efficacy of vagus nerve stimulation in the treatment of depression. Expert Rev Neurother. 2010;10:87–92. doi: 10.1586/ern.09.138. [DOI] [PubMed] [Google Scholar]
- 30.Li TT, Wang ZJ, Yang SB, et al. Transcutaneous electrical stimulation at auricular acupoints innervated by auricular branch of vagus nerve pairing tone for tinnitus: study protocol for a randomized controlled clinical trial. Trials. 2015;16:101. doi: 10.1186/s13063-015-0630-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.American Association of Neurological Surgeons Vagus Nerve Stimulation. 2015. [Accessed September 29, 2015]. Available at: http://www.aans.org/patient%20information/conditions%20and%20treatments/vagus%20nerve%20stimulation.aspx.
- 32.Kreuzer PM, Landgrebe M, Husser O, et al. Transcutaneous vagus nerve stimulation: retrospective assessment of cardiac safety in a pilot study. Front Psychiatry. 2012;3:1–7. doi: 10.3389/fpsyt.2012.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Frangosa E, Ellrich J, Komisaruka BR. Non-invasive access to the vagus nerve central projections via electrical stimulation of the external ear: fMRI evidence in humans. Brain Stimul. 2015;8:624–636. doi: 10.1016/j.brs.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao YX, He W, Jing XH, et al. Transcuatenous auricular vagus nerve stimulation protects endotoxemic rat from lipopolysacchardie-induced inflammation. Evid Based Complement Alternat Med. 2012;2012:627023. doi: 10.1155/2012/627023. [DOI] [PMC free article] [PubMed] [Google Scholar]