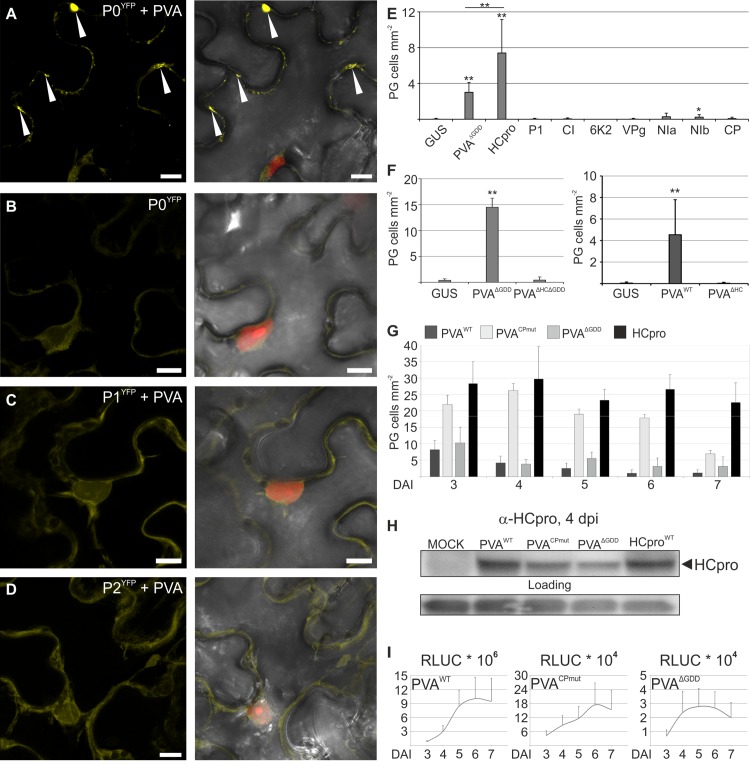
Fig 1. HCpro, the potyviral suppressor of RNA silencing, is the protein responsible for PVA-induced granules (PGs).
P0YFP was co-expressed with PVAWT (A) and alone (B), and P1YFP (C) and P2YFP (D) were expressed with PVAWT in Nicotiana benthamiana leaves using agroinfiltration and imaged by confocal microscopy three days later. RFP was expressed in A-D to visualize the nucleus. In (A- D) the left panel shows the YFP signal and the right panel an overlay view of YFP, nuclear RFP and the bright field (BF) as Z-stack projections. Arrowheads point out P0YFP-labeled PGs in (A). Scale bar; 10 μm. (E) Frequency of cells/mm2 containing PGs in leaves expressing P0YFP together with PVA proteins P1, HCpro, CI, 6K2, VPg, NIa, NIb or CP. PVAΔGDD was expressed as a positive and GUS as a negative control for PG induction. (F) Frequency of cells/mm2 containing PGs in leaves expressing P0YFP together with GUS, PVAΔGDD or PVAΔHCΔGDD (left panel) or GUS, PVA or PVAΔHC (right panel). (G) Frequency of cells/mm2 showing PGs in leaves expressing P0YFP with PVAWT, PVACPmut, PVAΔGDD or HCpro plotted as a function of time, (H) a western blot analysis of HCpro accumulation in PVAWT, PVACPmut, PVAΔGDD and HCpro samples using anti-HCpro PAb four days post infiltration and (I) virus derived RLUC activities analyzed in the same samples as in (H), again plotted as a function of time (days post infiltration). Modified PVA infectious cDNA constructs used in this study are schematically presented in (S1 Fig). All quantitative data is presented as means and the error bars indicate the standard deviations. (p < 0.01 **, p < 0.05 *).