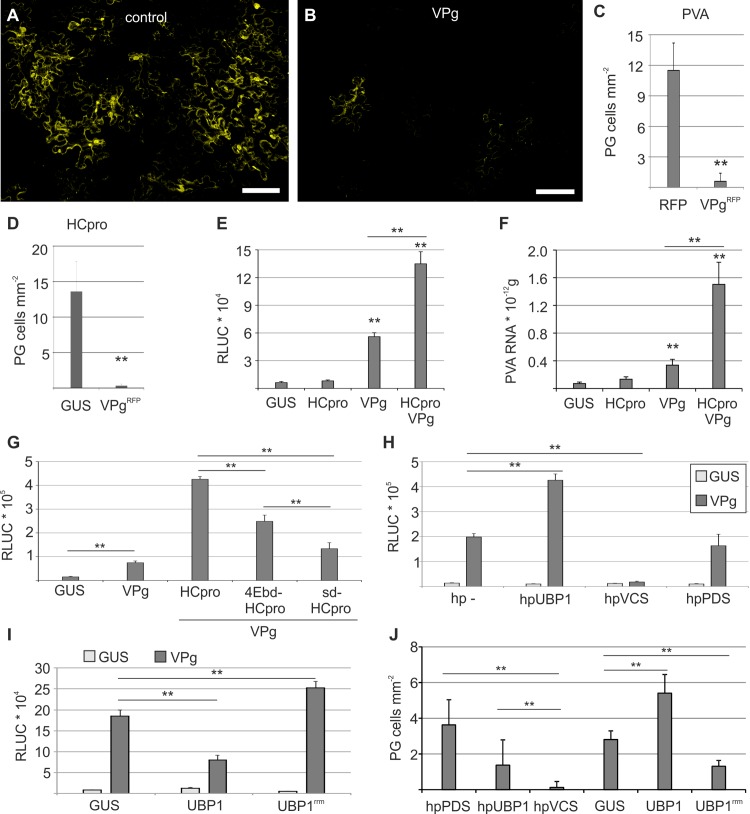
Fig 5. Viral protein VPg together with HCpro and other PG components regulates viral translation.
P0YFP and PVACPmut were expressed to induce PGs in N. benthamiana leaves, and the effect of co-expressed RFP (A) or VPgRFP (B) on P0YFP-labeled PGs was examined by confocal microscopy three days later. RFP fluorescence was used to verify VPg and control expressions. Scale bar; 100 μm. (C—D) Frequency of cells/mm2 containing P0YFP-labeled PGs in leaf tissues during expression of either RFP or VPgRFP. PVACPmut served as an inducer of the PGs in (C) and HCpro in (D). (E—F) PVAΔGDD RLUC activities and RNA levels were determined during co-expression of the vRNA with GUS, HCpro, VPg, VPg and HCpro in N. benthamiana leaves at 3 DAI. (G) PVAΔHCΔGDD RLUC activity levels were determined during co-expression of vRNA with GUS, VPg, VPg and either HCpro, 4Ebd-HCpro or sd-HCpro in N. benthamiana leaves at 3 DAI. (H) The capacity of VPg to elevate PVAΔGDD translation was analyzed during RNA hairpin (hp)-induced silencing of UBP1 and VCS in N. benthamiana leaves. Empty non-recombined silencing vector (hp-) and phytoene desaturase (PDS) hp-constructs were used as controls. VPg or GUS was expressed with PVAΔGDD in the different silencing backgrounds and RLUC activity was determined as a reporter of viral protein expression at 5 DAI. (I) The capacity of UBP1 and UBP1rrm to affect VPg promoted PVAΔGDD RLUC translation was analyzed. Here, GUS or VPg were co-expressed with PVAΔGDD during ectopic expression of GUS, UBP1, or UBP1rrm mutant lacking RNA-binding domains. All quantitative data is presented as mean and the error bar indicates the standard deviation. (J) The effect of UBP1 and VCS silencing and the effect of UBP1rrm overexpression on PVA-induced PG formation was analyzed by determining the frequency of cells/mm2 showing PGs in N. benthamiana leaves at 3 DAI. (p < 0.01 **, p < 0.05 *).