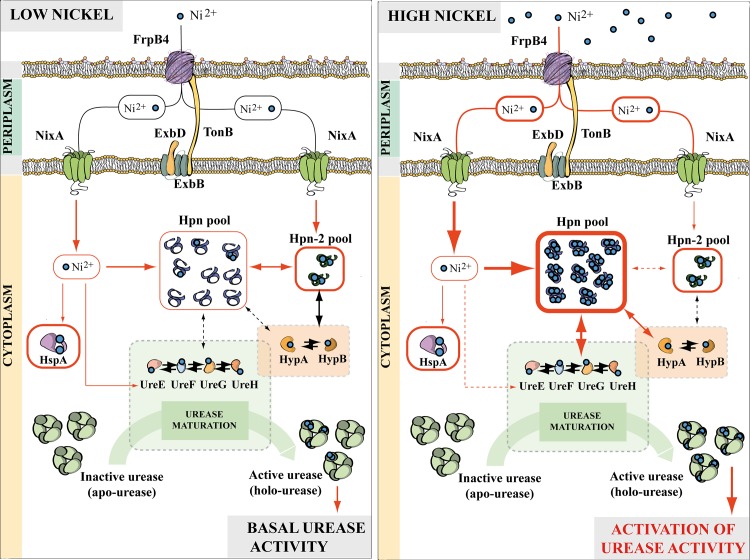
Fig 9. Model of the role of Hpn and Hpn-2 in nickel trafficking H. pylori.
In H. pylori, nickel is transported across the outer membrane by FrpB4, a TonB-dependent transporter. Once in the periplasm, uptake through the inner membrane occurs through the NixA permease (green). In the cytoplasm, nickel can follow several pathways, via the UreE/F/G/H or HypA/B accessory proteins dedicated to urease and/or hydrogenase maturation, the HspA co-chaperonine and Hpn or Hpn-2. (A) Under low nickel concentrations, and because Hpn is abundant, the Hpn cellular pool is not saturated with nickel, while Hpn-2, which is less abundant, can compete with Hpn (similar affinities for nickel). Nickel transfer from Hpn to the urease maturation machinery is low. This results in the “basal” urease activity level monitored in wild type H. pylori cells. (B) Under high nickel conditions, the Hpn protein pool stores more nickel and becomes saturated with this metal. Since Hpn-2 is less abundant, it rapidly becomes outcompeted. Under such conditions, interactions between Hpn and urease and/or hydrogenase maturation proteins enables Hpn-stored nickel transfer towards urease, leading to an increase in nickel incorporation, and enhanced urease activity.